-
PDF
- Split View
-
Views
-
Cite
Cite
Jesús Emiliano Sánchez-Garavito, Jorge Sanchez-Garcia, Daniel Olsen, Rami M Shorti, Fidel Lopez-Verdugo, Manuel I Rodriguez-Davalos, Liver resection for metastatic thyroid carcinoma. Case report and literature review, Journal of Surgical Case Reports, Volume 2020, Issue 9, September 2020, rjaa370, https://doi.org/10.1093/jscr/rjaa370
Close - Share Icon Share
Abstract
Liver resection for metastatic cancer has become the standard of care for specific groups of patients, including noncolorectal non-neuroendocrine liver metastases (NCNNELM). Liver metastasis from differentiated thyroid carcinoma is considered rare, with an approximated frequency of 0.5%. We present a case of metastatic papillary thyroid carcinoma (PTC) to the liver and literature review. Herein, we report a 72-year-old male that underwent formal left hepatectomy for 4.4 cm metastatic PTC generating left bile duct obstruction. Two months after, presented with multiple small lesions within the hepatic parenchyma and diffuse ductal dilatation of the right biliary system. Therefore, treated with a percutaneous biliary drain placement without complications. In a patient diagnosed with initial Stage II PTC, undergoing total thyroidectomy 10 years before presenting to the clinic. Bearing over a decade of treatments for local and distal recurrences. We believe approaching strategies for this specific disease should be developed to establish standard management.
INTRODUCTION
Liver resection for metastatic disease has become the standard of care for certain selected groups of patients [1]. However, the role of liver resection for noncolorectal non-neuroendocrine liver metastases (NCNNELM) is not defined for all types of primary tumors, due to the lack of sufficient data [2, 3]. The incidence of liver metastases from differentiated thyroid carcinoma (DTC; follicular- (FTC) and papillary-thyroid carcinoma (PTC)) are considered rare. This study aims to report a case of metastatic PTC to the liver and literature review.
CASE REPORT
A 72-year-old man, with no history of liver disease and normal body mass index, presented to our liver center with Stage IV PTC. Paternal history background of gastric cancer. In 2009, he underwent total thyroidectomy with modified radical right neck dissection and thyroid replacement after surgery due to an initial Stage II (pT2, N1b, M0) MACIS low risk (6.86), AGES (4.7) and AMES high-risk PTC diagnosis. In 2010, he received radioactive iodine ablation (RAI) I-131. Two years later, he had right neck recurrence treated with resection. At that time, the patient intended to undergo another round of I-131 failing the thymogen stimulation, thus considered iodine refractory. Afterward, he had multiple local and distal recurrent disease treated with neck dissections, mediastinal and right paratracheal resection, as well as a left craniotomy and tumor resection in 2018. All tumor resections reported metastatic PTC. In 2019, a positron emission tomography-computed tomography (CT) displayed recurrent disease on the right thyroid bed, left lower neck, right superior mediastinum, bilateral hilar regions and in the left lobe of his liver. Due to his adequate performance status (ECOG 0), Lenvatinib was offered as potential therapy, but the patient opted for observation.
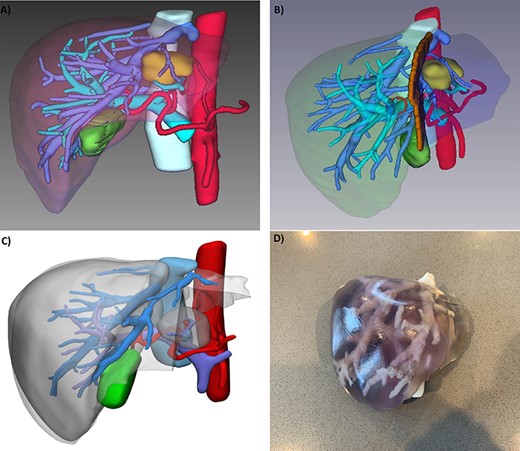
(A) 3D reconstruction of patient’s liver, tumor (yellow), arteries (red), portal vein branches (light blue), hepatic veins (blue); (B) potential transection plane; (C) digital segmentation for 3D printin; and (D) 3D printed model.
Six months later, he manifested epigastric discomfort and pruritus. Magnetic resonance imaging (MRI) showed a 2.8 × 2.4 cm liver mass on segments III and IV inducing left biliary system obstruction. It was decided to proceed with liver resection followed with postoperative Lenvatinib treatment. Presurgical workup included a triphasic CT scan for 3D reconstruction and printing (Intelligent/Interactive Qualitative and Quantitative Analysis, (Fig. 1), showing a 3.8 cm mass on the previously described location. The patient underwent laparoscopic exploration, converted to a formal left hepatectomy (I–IV). Pathology reported a 4.4 × 4.3 × 3.5 metastatic PTC (thyroglobulin and TTF-s1 positive) (Fig. 2). Two months after surgery, the patient presented to the emergency department with jaundice and diarrhea. MRI showed multiple (n = 10) lesions within the remaining hepatic parenchyma, the largest measuring 1 cm in diameter, causing diffuse ductal dilatation of the remaining right biliary system. A percutaneous biliary drain was placed after a failed endoscopic retrograde cholangiopancreatography.
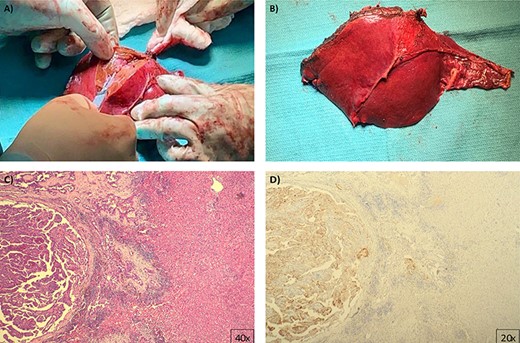
Pathology images (A) and (B) gross macroscopic pictures of excised specimen, an H&E stained slide (C) shows classic histologic features of papillary thyroid carcinoma confirmed by immunohistochemistry for thyroglobulin (D).
Postoperative Lenvatinib was previously discussed, but it was not fully covered by insurance. Even though his family would have covered out-of-pocket expenses, the patient had significant concerns regarding potential toxicities and lower quality of life with no guarantee of benefit. Ultimately, the patient and his family opted for hospice care, where he passed within 6 months from intervention without adjuvant therapy.
DISCUSSION
Several cases were reported before the acceptance of RAI and/or tyrosine kinase inhibitors (TKIs). The largest systematic review of NCNNELM includes almost 8000 patients [4]. Other NCNNELM cohorts with a combined sample size of over 8457 patients, have reported an incidence of 10 patients with thyroid carcinoma and/or head–neck tumors (Table 1) [2–5]. Meanwhile, Madani et al. [6] reported the largest systematic review of DTC distant metastases cohort with 492 patients and a frequency of 8% to the liver, however, management was not specified. Other cohorts as Hirsch et al. [7], Dinneen et al. [8] and Farina et al. [10] reported over 100 patients each with DTC distant metastasis and only seven patients with liver metastasis. Other cohorts and/or case reports are summarized in Table 2 [6–10]. In our patient, liver resection was intended to prevent recurrent cholangitis due to tumor obstruction and to reduce tumor burden followed by TKIs. However, the latter could not be performed due to the high costs and toxicity concerns of TKIs. We believe that an increased adoption of active surveillance may translate into an increase in long-term metastatic disease, like our patient had after 10 years of initial treatment. Therefore, strategies should be developed to establish standard management.
| Study . | Number of patients . | Frequency of thyroid metastasis . |
|---|---|---|
| Hoffmann et al. | 150 | One patient |
| Wakabayashi et al. | 205 | One patient (follicular thyroid carcinoma) |
| Fitzgerald et al. (Systematic review) | 7857 | A medullary thyroid cancer, a Hürtle cell cancer two patients |
| Lucchese et al. | 245 | Five patients with thyroid and/or head and neck tumors (not specified if it was PTC) |
| Study . | Number of patients . | Frequency of thyroid metastasis . |
|---|---|---|
| Hoffmann et al. | 150 | One patient |
| Wakabayashi et al. | 205 | One patient (follicular thyroid carcinoma) |
| Fitzgerald et al. (Systematic review) | 7857 | A medullary thyroid cancer, a Hürtle cell cancer two patients |
| Lucchese et al. | 245 | Five patients with thyroid and/or head and neck tumors (not specified if it was PTC) |
| Study . | Number of patients . | Frequency of thyroid metastasis . |
|---|---|---|
| Hoffmann et al. | 150 | One patient |
| Wakabayashi et al. | 205 | One patient (follicular thyroid carcinoma) |
| Fitzgerald et al. (Systematic review) | 7857 | A medullary thyroid cancer, a Hürtle cell cancer two patients |
| Lucchese et al. | 245 | Five patients with thyroid and/or head and neck tumors (not specified if it was PTC) |
| Study . | Number of patients . | Frequency of thyroid metastasis . |
|---|---|---|
| Hoffmann et al. | 150 | One patient |
| Wakabayashi et al. | 205 | One patient (follicular thyroid carcinoma) |
| Fitzgerald et al. (Systematic review) | 7857 | A medullary thyroid cancer, a Hürtle cell cancer two patients |
| Lucchese et al. | 245 | Five patients with thyroid and/or head and neck tumors (not specified if it was PTC) |
| Study . | Number of patients . | Liver resection . | RAI . | TKI . | Pathology diagnosis . | Survival . |
|---|---|---|---|---|---|---|
| Dinneen et al. | 100 (1% with liver metastasis) | 12% underwent surgery (not specified) | Yes (31%) | No | PTC | Overall survival rates at 5, 10 and 15 years were 37%, 24% and 20%, respectively |
| Hirsch et al. | 138 (3.6% with liver metastasis) | One liver resection | Yes (96.4%) | No | PTC follicular variant (66.7%), FTC (13.8%), poorly differentiated thyroid cancer (10.9%) and intermediate-risk tumors (8.7%) | One patient with liver metastases was disease-free at last follow-up 40.6% have died during the study years, disease-specific mortality rate was 23.2% |
| Zunino et al. | 36 (8% with liver metastasis) | Surgery not specified | Yes | Yes | 72.2% with PTC, 27.7% with FTC | Survival in patients with liver metastases ranged from 4.75 to 28 months in patients who were not treated and those who received TKI, respectively |
| Madani et al. | 492 (8% with liver metastasis) | Surgical metastasectomy | Yes | Yes (3%). | 57% with PTC, 39% with FTC, four with Hürthle-cell, 43% with FTC (liver specific) | Mean overall survival after diagnosis 60 months |
| Farina et al. | 103 (1% with liver metastasis) | No | Yes | Yes | PTC | 6-month survival in patient with liver metastases |
| Study . | Number of patients . | Liver resection . | RAI . | TKI . | Pathology diagnosis . | Survival . |
|---|---|---|---|---|---|---|
| Dinneen et al. | 100 (1% with liver metastasis) | 12% underwent surgery (not specified) | Yes (31%) | No | PTC | Overall survival rates at 5, 10 and 15 years were 37%, 24% and 20%, respectively |
| Hirsch et al. | 138 (3.6% with liver metastasis) | One liver resection | Yes (96.4%) | No | PTC follicular variant (66.7%), FTC (13.8%), poorly differentiated thyroid cancer (10.9%) and intermediate-risk tumors (8.7%) | One patient with liver metastases was disease-free at last follow-up 40.6% have died during the study years, disease-specific mortality rate was 23.2% |
| Zunino et al. | 36 (8% with liver metastasis) | Surgery not specified | Yes | Yes | 72.2% with PTC, 27.7% with FTC | Survival in patients with liver metastases ranged from 4.75 to 28 months in patients who were not treated and those who received TKI, respectively |
| Madani et al. | 492 (8% with liver metastasis) | Surgical metastasectomy | Yes | Yes (3%). | 57% with PTC, 39% with FTC, four with Hürthle-cell, 43% with FTC (liver specific) | Mean overall survival after diagnosis 60 months |
| Farina et al. | 103 (1% with liver metastasis) | No | Yes | Yes | PTC | 6-month survival in patient with liver metastases |
| Study . | Number of patients . | Liver resection . | RAI . | TKI . | Pathology diagnosis . | Survival . |
|---|---|---|---|---|---|---|
| Dinneen et al. | 100 (1% with liver metastasis) | 12% underwent surgery (not specified) | Yes (31%) | No | PTC | Overall survival rates at 5, 10 and 15 years were 37%, 24% and 20%, respectively |
| Hirsch et al. | 138 (3.6% with liver metastasis) | One liver resection | Yes (96.4%) | No | PTC follicular variant (66.7%), FTC (13.8%), poorly differentiated thyroid cancer (10.9%) and intermediate-risk tumors (8.7%) | One patient with liver metastases was disease-free at last follow-up 40.6% have died during the study years, disease-specific mortality rate was 23.2% |
| Zunino et al. | 36 (8% with liver metastasis) | Surgery not specified | Yes | Yes | 72.2% with PTC, 27.7% with FTC | Survival in patients with liver metastases ranged from 4.75 to 28 months in patients who were not treated and those who received TKI, respectively |
| Madani et al. | 492 (8% with liver metastasis) | Surgical metastasectomy | Yes | Yes (3%). | 57% with PTC, 39% with FTC, four with Hürthle-cell, 43% with FTC (liver specific) | Mean overall survival after diagnosis 60 months |
| Farina et al. | 103 (1% with liver metastasis) | No | Yes | Yes | PTC | 6-month survival in patient with liver metastases |
| Study . | Number of patients . | Liver resection . | RAI . | TKI . | Pathology diagnosis . | Survival . |
|---|---|---|---|---|---|---|
| Dinneen et al. | 100 (1% with liver metastasis) | 12% underwent surgery (not specified) | Yes (31%) | No | PTC | Overall survival rates at 5, 10 and 15 years were 37%, 24% and 20%, respectively |
| Hirsch et al. | 138 (3.6% with liver metastasis) | One liver resection | Yes (96.4%) | No | PTC follicular variant (66.7%), FTC (13.8%), poorly differentiated thyroid cancer (10.9%) and intermediate-risk tumors (8.7%) | One patient with liver metastases was disease-free at last follow-up 40.6% have died during the study years, disease-specific mortality rate was 23.2% |
| Zunino et al. | 36 (8% with liver metastasis) | Surgery not specified | Yes | Yes | 72.2% with PTC, 27.7% with FTC | Survival in patients with liver metastases ranged from 4.75 to 28 months in patients who were not treated and those who received TKI, respectively |
| Madani et al. | 492 (8% with liver metastasis) | Surgical metastasectomy | Yes | Yes (3%). | 57% with PTC, 39% with FTC, four with Hürthle-cell, 43% with FTC (liver specific) | Mean overall survival after diagnosis 60 months |
| Farina et al. | 103 (1% with liver metastasis) | No | Yes | Yes | PTC | 6-month survival in patient with liver metastases |
The patient fought >10 years against this slow-growing cancer. This characteristic plus their high recurrence capability make these tumors arguably aggressive. Neoadjuvant chemotherapy should be considered to decrease tumor burden, though studies have shown that lesion growth during chemotherapy is associated with poor outcomes among patients with extrahepatic disease compared to those with controlled disease. Early detection and rapid referral to a hepatobiliary center are essential, as well as multidisciplinary management.
DECLARATIONS
This paper was approved by the Institutional Review Board and consent was signed. Research was performed in accordance with the Declaration of Helsinki.
Acknowledgment
None. No funding was received for this article.
AUTHORS’ CONTRIBUTIONS
J.E.S.G. and J.S.G. participated in performance of research, data acquisition and writing of the paper; D.O. participated in performance of research, data acquisition and critical review of the manuscript; R.S. participated in performance of research and data acquisition; F.L.V. participated in performance of research, data acquisition and manuscript review and editing; M.I.R.D participated in research design, project supervision, critical review and editing of manuscript; R.M.S. participated in imaging reconstruction/design and 3D printing.
CONFLICT OF INTEREST STATEMENT
None declared.



