-
PDF
- Split View
-
Views
-
Cite
Cite
Rachel Harwood, Roland Partridge, Joanne Minford, Sarah Almond, Paediatric abdominal pain in the time of COVID-19: a new diagnostic dilemma, Journal of Surgical Case Reports, Volume 2020, Issue 9, September 2020, rjaa337, https://doi.org/10.1093/jscr/rjaa337
Close - Share Icon Share
Abstract
The diagnostic uncertainty for children with abdominal pain has increased during the COVID-19 pandemic with the additional consideration of both COVID-19 and paediatric inflammatory multisystem syndrome—temporally associated with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (PIMS-TS) alongside appendicitis, mesenteric adenitis and other less common causes of abdominal pain. We describe the cases of two children who presented with severe abdominal pain, non-bilious vomiting and high temperatures during the UK’s first peak of the COVID-19 pandemic. Laboratory and abdominal ultrasound features were similar for both children but symptom progression in combination with cross-sectional abdominal imaging enabled differentiation between PIMS-TS and appendicitis with concurrent COVID-19. These cases highlight the importance of regular clinical review, multidisciplinary working and the utility of early cross-sectional imaging to determine the underlying condition.
INTRODUCTION
Pandemic COVID-19 has changed the approach to established care pathways for many conditions. The assessment and management of abdominal pain, the most common paediatric acute condition seen by surgeons, has been turned on its head. It is now well established that the differential diagnosis of abdominal pain includes severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection [1] and paediatric inflammatory multisystem syndrome—temporally associated with SARS-CoV-2 (PIMS-TS) which presents with abdominal pain in ~50% of affected children [2].
CASE SERIES
We report two children admitted to our institution with abdominal pain during the early pandemic.
Patient A, a 14-year-old girl with no underlying medical conditions, presented with 4 days of central abdominal pain which migrated to the right iliac fossa, non-bilious vomiting and high temperatures. Of note, her mother, a healthcare worker, had symptoms of shortness of breath and high temperature 2 weeks previously but had not been swabbed for COVID-19.
Examination findings revealed the patient to be mildly tachycardic but normotensive. She was apyrexial and had abdominal tenderness centrally and in the right iliac fossa and was reported to be Rosving’s positive. The patient had markedly raised C-reactive protein (CRP) and lymphopenia along with a high D-Dimer and elevated ferritin. Her transaminases were also noted to be mildly elevated (Table 1). Abdominal ultrasound scan (A-USS) demonstrated mesenteric adenitis without free fluid and chest X-ray was unremarkable. Within 12 hours of admission she developed a palmar rash, followed by shock. Urgent computed tomography (CT) scan of the chest and abdomen revealed diffuse intra- and interlobar thickening consistent with SARS-CoV-2 infection and no intra-abdominal pathology (Fig. 1A and B). She was SARS-CoV-2 negative on reverse transcriptase polymerase chain reaction. Patient A was treated successfully for PIMS-TS with supportive care including antibiotics until the blood cultures were negative and the interleukin-1 antagonist anakinra [3]. The patient underwent an echocardiogram on Day 8 and was found to have left coronary artery ectasia and has ongoing follow-up of this important sequelae of PIMS-TS.
Haematological and biochemical markers taken at admission of Patient A and B
| . | Patient A . | Patient B . |
|---|---|---|
| Haematology | ||
| White cell count | 8.23 × 109/L | 12.74 × 109/L |
| Neutrophils | 7.42 × 109/L | 9.79 × 109/L |
| Lymphocytes | 0.14 × 109/L | 1.51 × 109/L |
| Haemaglobin | 123 g/L | 100 g/L |
| D-Dimer | >4810 ng/ml (taken 2 days after admission) | 9466 ng/ml (taken 1 day after admission) |
| Biochemistry | ||
| Urea | 3.5 mmol/L | 2.5 mmol/L |
| Creatinine | 54 umol/L | 25 umol/L |
| Bilirubin | 31 umol/L | 7 umol/L |
| ALT | 156 iu/L | 8 iu/L |
| AST | 166 iu/L | 24 iu/L |
| C-reactive protein | 242 mg/L | 261.7 mg/L |
| Ferritin | 328 ng/ml (taken 2 days after admission) | 104 ng/ml (taken 1 day after admission) |
| . | Patient A . | Patient B . |
|---|---|---|
| Haematology | ||
| White cell count | 8.23 × 109/L | 12.74 × 109/L |
| Neutrophils | 7.42 × 109/L | 9.79 × 109/L |
| Lymphocytes | 0.14 × 109/L | 1.51 × 109/L |
| Haemaglobin | 123 g/L | 100 g/L |
| D-Dimer | >4810 ng/ml (taken 2 days after admission) | 9466 ng/ml (taken 1 day after admission) |
| Biochemistry | ||
| Urea | 3.5 mmol/L | 2.5 mmol/L |
| Creatinine | 54 umol/L | 25 umol/L |
| Bilirubin | 31 umol/L | 7 umol/L |
| ALT | 156 iu/L | 8 iu/L |
| AST | 166 iu/L | 24 iu/L |
| C-reactive protein | 242 mg/L | 261.7 mg/L |
| Ferritin | 328 ng/ml (taken 2 days after admission) | 104 ng/ml (taken 1 day after admission) |
Haematological and biochemical markers taken at admission of Patient A and B
| . | Patient A . | Patient B . |
|---|---|---|
| Haematology | ||
| White cell count | 8.23 × 109/L | 12.74 × 109/L |
| Neutrophils | 7.42 × 109/L | 9.79 × 109/L |
| Lymphocytes | 0.14 × 109/L | 1.51 × 109/L |
| Haemaglobin | 123 g/L | 100 g/L |
| D-Dimer | >4810 ng/ml (taken 2 days after admission) | 9466 ng/ml (taken 1 day after admission) |
| Biochemistry | ||
| Urea | 3.5 mmol/L | 2.5 mmol/L |
| Creatinine | 54 umol/L | 25 umol/L |
| Bilirubin | 31 umol/L | 7 umol/L |
| ALT | 156 iu/L | 8 iu/L |
| AST | 166 iu/L | 24 iu/L |
| C-reactive protein | 242 mg/L | 261.7 mg/L |
| Ferritin | 328 ng/ml (taken 2 days after admission) | 104 ng/ml (taken 1 day after admission) |
| . | Patient A . | Patient B . |
|---|---|---|
| Haematology | ||
| White cell count | 8.23 × 109/L | 12.74 × 109/L |
| Neutrophils | 7.42 × 109/L | 9.79 × 109/L |
| Lymphocytes | 0.14 × 109/L | 1.51 × 109/L |
| Haemaglobin | 123 g/L | 100 g/L |
| D-Dimer | >4810 ng/ml (taken 2 days after admission) | 9466 ng/ml (taken 1 day after admission) |
| Biochemistry | ||
| Urea | 3.5 mmol/L | 2.5 mmol/L |
| Creatinine | 54 umol/L | 25 umol/L |
| Bilirubin | 31 umol/L | 7 umol/L |
| ALT | 156 iu/L | 8 iu/L |
| AST | 166 iu/L | 24 iu/L |
| C-reactive protein | 242 mg/L | 261.7 mg/L |
| Ferritin | 328 ng/ml (taken 2 days after admission) | 104 ng/ml (taken 1 day after admission) |
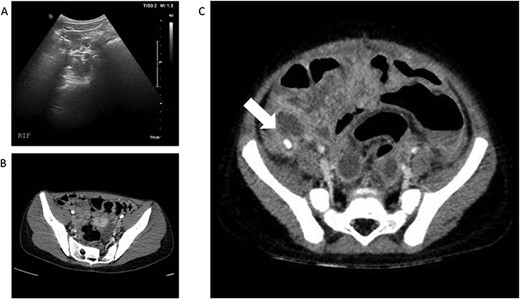
(A) and (B)—Case 1: abdominal ultrasound scan and computed tomography (CT) scan of abdomen, (C)—Case 2: CT scan of abdomen; white arrow shows faecolith with inflamed appendix and surrounding collections.
Patient B, a 3-year-old boy, presented similarly with a 4 day history of abdominal pain, non-bilious vomiting and high temperatures. He had normal observations for his age but had abdominal distension and a tender lower abdomen. He also had a raised CRP along with an elevated D-Dimer and raised Ferritin (Table 1). A-USS and echocardiogram performed on Day 1 of admission were normal and he was SARS-CoV-2 positive on nasopharyngeal swab. Intravenous piperacillin and tazobactam were commenced to treat for possible concurrent sepsis and a presumptive diagnosis of PIMS-TS was made. Over the subsequent 12 hours he deteriorated with worsening pain and vomiting but it was noted that his CRP had reduced from 261 mg/L to 171 mg/L. Abdominal CT scan was undertaken and revealed perforated appendicitis with intra-abdominal collections (Fig. 1C). He underwent open appendicectomy without immediate complication and recovered well. He was treated for a superficial wound infection 7 days post-operatively with oral antibiotics.
DISCUSSION
These cases highlight the additional diagnostic uncertainty that exists during the COVID-19 pandemic. These two patients show that the initial clinical presentation of PIMS-TS and appendicitis with concurrent COVID-19 infection can be indistinguishable. D-Dimer and ferritin do not appear to be good markers to enable differentiation between PIMS-TS and alternative infective pathologies, although they may be of use to determine the severity of PIMS-TS. Early signals that Patient B had an alternative diagnosis to PIMS-TS include his normal liver function tests and normal lymphocyte count, along with a normal echocardiogram, although all of these are possible with a diagnosis of PIMS-TS. Improvement in inflammatory markers after the commencement of antibiotics pointed towards an infective diagnosis rather than PIMS-TS and the combination of these features led to a high clinical suspicion of an alternative diagnosis.
A-USS remains the mainstay of imaging for children with abdominal pain, avoiding the radiation of CT scan, but it is less sensitive (55% vs 98%) and specific (85% vs 100%) than CT in diagnosing appendicitis [4]. A recent series showed that 4/8 (50%) children with PIMS-TS who presented with abdominal pain had a CT scan after A-USS was unable to locate the appendix or exclude acute appendicitis [5]. The children with PIMS-TS were found to have terminal ileitis, often with free intra-peritoneal fluid in the right iliac fossa.
Although the maxim of Occam’s razor remains relevant, in the context of pandemic infection it is crucial to recognise that COVID-19 can occur with other pathologies. Conditions with well-defined treatments, such as appendicitis, can be easily missed, leading to significant morbidity. Judicious, repeated clinical assessment, multi-speciality teamworking and a low threshold for cross-sectional abdominal imaging are recommended to enable differentiation between PIMS-TS, COVID-19 and acute appendicitis in unwell children with abdominal pain and unremarkable abdominal ultrasonography.
ACKNOWLEDGEMENTS
We would like to acknowledge the input of other members of the multidisciplinary team in the care of these patients including Dr C Pain, Dr S Felenstein, Dr G Cleary, Dr S Mayell, Dr K Conrad, Dr S Harave, Dr P Duong, Dr I Sinha, Dr D Porter and Dr C Hedrich.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
None.



