-
PDF
- Split View
-
Views
-
Cite
Cite
Derrick Acheampong, Percy Boateng, Colonic diverticulitis following open-heart surgery: a case report of an unusual postoperative gastrointestinal complication, Journal of Surgical Case Reports, Volume 2020, Issue 6, June 2020, rjaa186, https://doi.org/10.1093/jscr/rjaa186
Close - Share Icon Share
Abstract
Diverticulitis, though a common gastrointestinal disease, is rare following open-heart surgery. There is insufficient data regarding its incidence and management post-cardiac surgery. Especially in patients with atypical presentation, diagnosis and management can be challenging. This case outlines one such atypical diverticulitis case in which a 57-year-old female patient developed perforated diverticulitis with pelvic abscess accumulation following left ventricular aneurysm (LVA) repair. Diagnosis, appropriate management and treatment approaches are discussed. Cardiac surgeons should consider the possibility of diverticulitis in patients reporting nonspecific abdominal pain following cardiac surgery to ensure early diagnosis and institution of appropriate treatment to prevent associated adverse outcomes.
INTRODUCTION
Improvements in operative techniques and perioperative care in cardiac surgery have enhanced surgical outcomes; however, postoperative gastrointestinal (GI) complications following cardiac surgery continue to carry significant burden [1]. GI complications range from 0.3 to 5.5%, but associated mortalities have been reported as high as 87% [1]. Among GI complications following cardiac surgery, diverticulitis is rarely encountered. Here, we report the case of a 57-year-old woman who developed perforated diverticulitis after left ventricular aneurysm (LVA) repair. We will use this case report to outline diagnosis and management for diverticulitis after open-heart surgery.
CASE REPORT
A 57-year-old woman underwent elective resection and patch repair of LVA with cardiopulmonary bypass (CPB) without intraoperative complications. Total CPB time and cross clamp time were 165 and 139 minutes, respectively. Prophylactic antibiotics were given per routine and discontinued 24 hours postoperatively.
Immediate postoperative course was uneventful until postoperative day (POD)-5 when she experienced multiple watery stools, diffuse abdominal pain, fever (102.6F) and leukocytosis (23000/UL). Clostridium difficile infection was ruled out with a negative stool toxin assay. Her abdominal exam remained nonspecific until POD-6 when she had bilious emesis. A computed tomography (CT) scan revealed perforated diverticulitis with pelvic abscess and multiple colonic diverticulitis with an associated small bowel obstruction (SBO) (Images 1–4). No prior history of diverticulitis was reported.
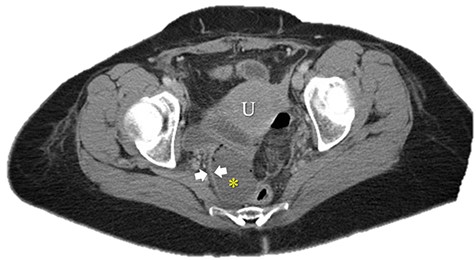
Axial image of pelvic collection. U: uterus; *: pelvic abscess cavity; White arrows: rim-enhancing pelvic abscess cavity.
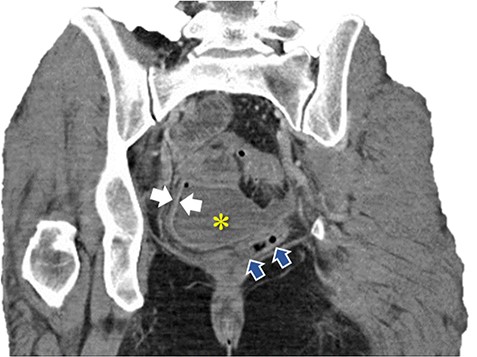
Coronal image of pelvic abscess cavity. *: pelvic abscess cavity; White arrows: rim-enhancing pelvic abscess cavity; Blue arrows: collapsed sigmoid colon with multiple diverticuli.
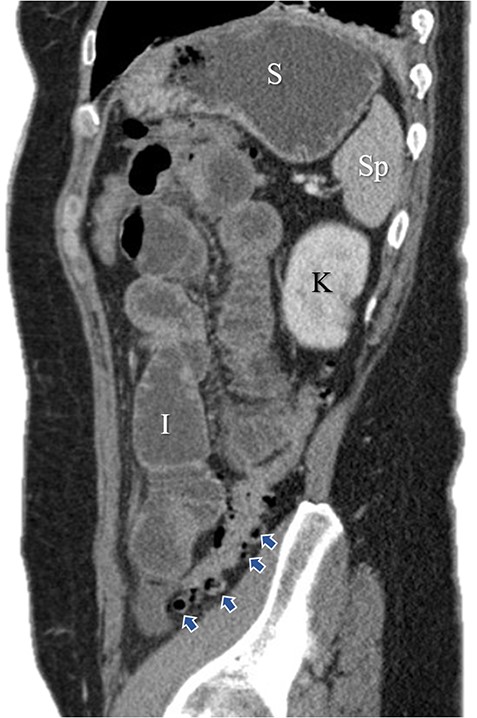
Sagittal image of colonic diverticuli. S: stomach; Sp: spleen; K: kidney; I: ileum; Blue arrows: multiple colonic diverticuli.
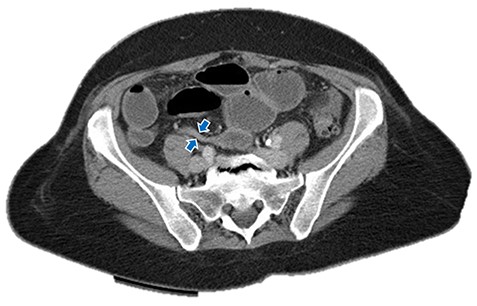
Small bowel obstruction showing air-fluid levels with transition point in the distal ileum. Blue arrows: small bowel obstruction with transition point in distal ileum.
She was managed with bowel rest, intravenous fluids, nasogastric tube suction and analgesics and empirically started on broad-spectrum gram negative and anaerobic antibiotics. Her pelvic abscess was drained percutaneously with one trans-gluteal drain. She improved significantly with some return of GI function on POD-7. Because of persistent leukocytosis, a follow-up CT scan was done 5 days post-drain placement which showed marked improvement of the pelvic abscess (Image 5). She was discharged home on ceftriaxone and oral metronidazole on POD-16 to complete a total of 14 days antibiotic treatment. The drain was removed before discharge. She reported no abdominal symptoms during her 1-month follow-up.
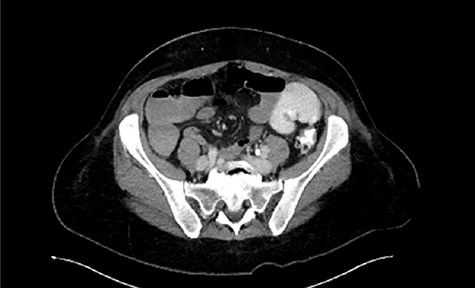
Follow-up CT showing marked improvement of pelvic abscess collection.
This study was reviewed and approved by the Mount Sinai Hospital Institutional Review Board.
DISCUSSION
GI complications after open-heart surgery, though rare, carry significant morbidity and mortality burden [1]. Commonly observed GI complications after open-heart surgery include mesenteric ischemia, upper GI bleeding, pancreatitis, cholecystitis and ileus [1]. Diverticulitis following open-heart surgery is rarely discussed. This case highlights the importance of considering diverticulitis as a potential life-threatening complication following heart surgery.
The pathogenesis of diverticulitis and its epidemiology is well described [2, 3]. The precipitant for diverticulitis after cardiac surgery is not well-known; however, we believe that perioperative unfavorable hemodynamic changes, vasopressor use, splanchnic hypoperfusion during CPB and possibly prolonged CPB time may all be contributing factors that lead to tissue ischemia and a resultant diverticular infection.
Diverticulitis often presents with fever and constant left lower quadrant abdominal pain; however, some patients have atypical symptoms of tachycardia, hypotension, diarrhea, diffuse abdominal pain and distention [2, 3]. These atypical patients usually present a diagnostic and therapeutic challenge and often tend to progress to complicated diverticulitis by the time of diagnosis. And since early diagnosis of diverticulitis is essential for better outcomes [2, 3], prognosis remains grim and mortality high in such patients, making it important for cardiac surgeons to develop a high index of suspicion for diverticulitis in the appropriate patient demographic with abdominal pain following cardiac surgery.
The usual laboratory workup for infection is neither sensitive nor specific for diverticulitis [2, 3]. In the setting of symptoms and signs of infection, a localizing abdominal exam is helpful to guide and initiate therapy; however, the most sensitive diagnostic approach is an abdominal CT scan since it provides an accurate diagnosis and extent of disease severity [2, 3].
Understanding the disease grade helps manage diverticulitis. Several classifications for grading diverticulitis exist, but the most widely used is Hinchey classification [4]. Hinchey classified diverticulitis into pericolic abscess (Hinchey I); pelvic, abdominal or retroperitoneal abscess (Hinchey II); purulent peritonitis (Hinchey III); and fecal peritonitis (Hinchey IV) [4]. Higher Hinchey classes (III and IV) are more complicated and require extensive management. Our patient had Hinchey II diverticulitis.
In general, management of diverticulitis involves bowel rest, fluid replacement, antibiotic therapy, percutaneous drainage, colon resection and one- or two-stage anastomosis, depending on severity [2–5]. Patients with Hinchey I and II diverticulitis are usually managed medically with bowel rest, fluid replacement and antibiotic therapy, while Hinchey III and IV patients usually undergo emergent definitive sigmoid resection with primary anastomosis +/− protective ileostomy formation or Hartmann’s procedure [5]. Still, should medical management fail in Hinchey I and II diverticulitis, more aggressive surgical management should be considered.
CONCLUSION
Cardiac surgeons should consider the possibility of diverticulitis in patients reporting nonspecific abdominal pain following cardiac surgery to ensure early diagnosis and institution of appropriate treatment to prevent associated adverse outcomes.



