-
PDF
- Split View
-
Views
-
Cite
Cite
Irena Stefanova, Jeremy R Huddy, John Richardson, A rare case of acute congestive ischaemic colitis related to combined superior and inferior mesenteric arteriovenous malformations, Journal of Surgical Case Reports, Volume 2020, Issue 4, April 2020, rjaa083, https://doi.org/10.1093/jscr/rjaa083
Close - Share Icon Share
Abstract
Visceral arteriovenous malformations (AVMs) are extremely rare with only a few cases described within the literature. To date, no cases of ischaemic colitis related to arteriovenous malformations affecting both superior and inferior mesenteric arteries have been reported. We report the first case of acute ischaemic colitis caused by venous congestion and reduced arterial flow due to combined AVMs in the territory of superior and inferior mesenteric arteries in a 51-year-old patient. After a multidisciplinary meeting, interventional radiology embolization was considered to be of unlikely benefit due to extensive varicosities; therefore, surgical treatment in the form of open subtotal colectomy and end ileostomy was performed. This case report demonstrates the severity and the complexity in the management of AVM-related ischaemic colitis, together with a review of the literature.
INTRODUCTION
Visceral arteriovenous malformations (AVMs) are an exceptionally rare cause of ischaemic colitis with few cases described within the literature [2, 3, 5, 8–10]. They can be congenital or acquired. To our knowledge, no cases of AVMs affecting both superior (SMA) and inferior (IMA) mesenteric arteries have been reported. We report the first case of ischaemic colitis caused by venous congestion and reduced arterial flow due to combined inferior and superior mesenteric AVMs.
CASE REPORT
A 51-year-old male patient presented to the emergency department with 24 hours of severe abdominal pain and rectal bleeding, as well as 4 months history of non-mucous diarrhoea with stool frequency of up to 12 episodes per day. He had a background of poorly differentiated adenocarcinoma of the ascending colon (T3N0M0), which was treated with laparoscopic right hemicolectomy 1 year prior to this presentation. His follow-up colonoscopy 6 months prior to admission demonstrated evidence of diverticular disease and colitis although biopsies were normal. On admission, the patient was haemodynamically stable and physical examination did not reveal signs of peritonism. Laboratory studies demonstrated a haemoglobin drop of 4 g/dl (15.9–10.8 g/dl) but no evidence of abnormal coagulation. Stool samples were negative for infectious causes. The diagnosis of ischaemic colitis was made by contrast-enhanced computed tomography (CT) scan with CT angiography, which revealed AVMs in the territory of SMA and IMA and acute massive congestive ischaemic colitis affecting remaining colon and rectum (Figs 1 and 2).
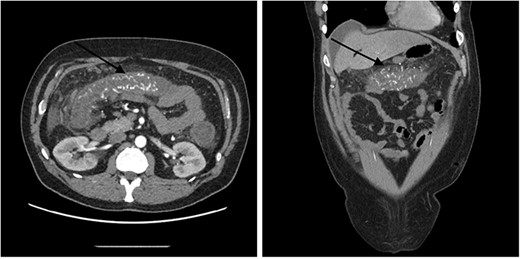
Contrast-enhanced abdominal CT images of superior mesenteric AVM causing acute congestive ischaemic colitis affecting transverse colon.
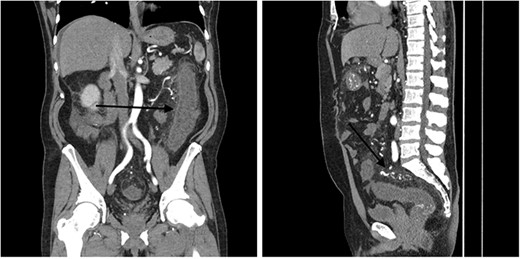
Contrast-enhanced abdominal CT images of inferior mesenteric AVM causing acute congestive ischaemic colitis affecting descending and sigmoid colon, and rectum.
Additionally, flexible sigmoidoscopy showed engorged erythematous colonic mucosa but no ulcerations or polyps (Fig. 3). All biopsies revealed normal mucosa.
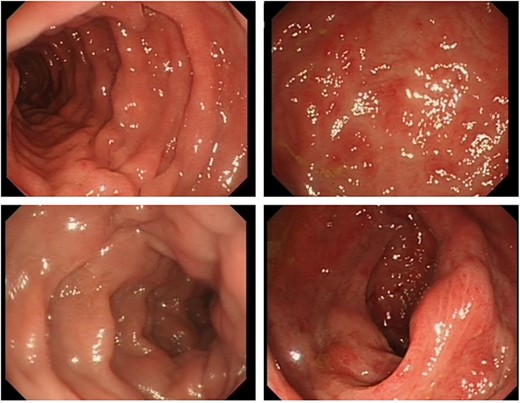
Flexible sigmoidoscopy images showing engorged erythematous appearance of the colonic mucosa. No ulcerations, polyps or blood was found.
In the first instance, medical management of colitis was initiated. This included high-dose intravenous corticosteroids and 5-aminosalicylic acid agents for 2 weeks. There was no significant improvement of symptoms. During his hospital stay, the patient required transfusion of four units packed red blood cells. Following unsuccessful medical treatment, surgical options were considered. Discussion at colorectal surgery multi-disciplinary meeting with the involvement of interventional radiologist concluded that embolization would be of unlikely benefit due to extensive varicosities, and decision for surgical treatment was made. The patient underwent open subtotal colectomy and end ileostomy based on the intraoperative findings (bluish discolouration of colon, oedematous mesocolon and appendices epiploicae, extensive varicosities—Figs 4 and 5). Rectal stump was left given the presence of collateral blood supply.
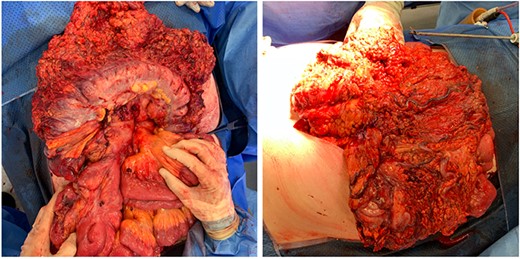
Intraoperative findings of bluish discolouration of transverse colon and extensive varicosities.
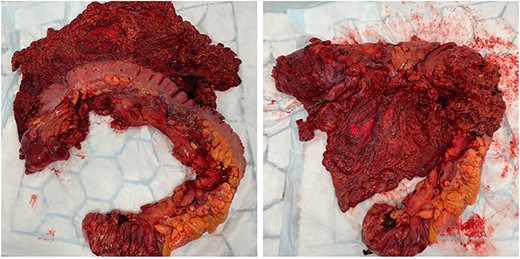
Intraoperative findings of oedematous appendices epiploicae and mesocolon.
Histopathological results confirmed the diagnosis. Detailed microscopic description included mucosa with foci of ulceration and necrosis, submucosa with marked oedema and congestion, but no evidence of crypt abscesses, dysplasia or malignancy. We observed a full patient recovery.
DISCUSSION
Ischaemic colitis (IC) is a common acute surgical presentation and the most prevalent cause of gastrointestinal ischaemia. IC is frequently classified as occlusive and non-occlusive with the latter being the predominant mechanism (95% of the cases) [1].
A number of factors predispose the colon to ischaemia and increase its vulnerability to hypoperfusion. These include reduced blood flow per 100 g of tissue compared to other areas of the gastrointestinal tract; presence of ‘watershed areas’ with limited collateralization such as the splenic flexure and the rectosigmoid junction [1]; and poor autoregulation during hypotension [2].
AVMs are an exceptionally rare cause of IC. Many authors use the terms AVM and arteriovenous fistula (AVF) interchangeably as their pathophysiological consequences are very similar [3]. Nevertheless, the congenital AVMs differ from the acquired AVFs which are caused by blunt or penetrating abdominal trauma, or have iatrogenic aetiology (e.g. surgical resection) [2]. The suggested mechanism through which an AVM causes IC is a combination of reduced arterial blood flow to the mucosa secondary to a steal phenomenon as blood bypasses the capillary bed, and congestive submucosal oedema due to venous hypertension [2].
AVMs more often involve the hepatic (45%), splenic (30%), SMA and gastroduodenal arteries. AVMs in the territory of the IMA are extremely rare [3]. In a detailed review from 2014, Athanasiou et al. identified 26 cases of AVM involving the IMA [3, 4]. Since then, there were only seven other cases [3, 5–10] described within the literature.
The reported mean age of presentation of congenital inferior mesenteric AVMs is 58 years. Typical presenting clinical features include abdominal pain, lower and upper gastrointestinal bleeding, abdominal mass, portal hypertension and IC [4].
Congestive IC is one of the most serious complications (50% of cases) of inferior mesenteric AVM [10]. The classic clinical picture includes diarrhoea, rectal bleeding and abdominal pain, which were all present in our case. The guidelines for investigating IC recommend the use of combination of diagnostic methods [1]. Multidetector computed tomography (MDCT) usually reveals bowel wall thickening and mesenteric stranding, which are non-specific signs. Severe ischaemia can progress to infarction and perforation. Peritonitis, pneumatosis, portal or mesenteric gas can be seen on MDCT. Endoscopic visualization usually reveals submucosal oedema, necrotic mucosa with ulcers or infarction, and this is confirmative of the diagnosis [3]. Angiography or magnetic resonance angiography can be valuable in demonstrating the precise anatomy of the AVM.
The treatment of inferior mesenteric AVMs is very complex and requires case-specific multidisciplinary approach. It involves either endovascular embolization or surgical intervention. Embolization is successful in most cases described in the literature either as a bridge prior to operative management or as a definitive treatment. It is thought to be safer and to reduce the risk of intraoperative blood loss. However, it carries the risk of ischemia and passage of embolization material into the portal circulation [4]. Furthermore, often collateral formation follows embolization if surgical intervention is not performed shortly after [5]. In our case, the severity of a combined inferior and superior mesenteric AVMs, and extensive varicosities led to inability to include endovascular embolization as an option for treatment. In this circumstance, the preferred treatment was surgery alone.
CONCLUSION
This is the first reported case of combined SMA and IMA AVMs and the second case demonstrating such severity of AVM-related IC mandating surgical treatment for symptom control as opposed to attempt of interventional radiology embolization prior. Due to this conditions’ complexity, a case-specific approach with involvement of medical, surgical and radiological expertise is essential.
CONFLICT OF INTEREST
None declared.
FUNDING
None.



