-
PDF
- Split View
-
Views
-
Cite
Cite
Yusuke Yamagishi, Masanori Okamoto, Yasuo Yoshimura, Munehisa Kito, Kaoru Aoki, Jun Takahashi, Continued growth of locally aggressive fibrous dysplasia of 22 years duration after reaching adulthood: a case report, Journal of Surgical Case Reports, Volume 2020, Issue 2, February 2020, rjz406, https://doi.org/10.1093/jscr/rjz406
Close - Share Icon Share
Abstract
Fibrous dysplasia generally stops growing when patients reach adulthood. Locally aggressive fibrous dysplasia is an extremely rare subtype of fibrous dysplasia that is characterized by progressive enlargement after bone maturation, cortical bone destruction and soft tissue invasion but without malignant transformation. At 50 years of age, a tumor was found in the rib of a patient. The tumor gradually enlarged over time and imaging findings suggested a malignant tumor. The case was further complicated by restrictive lung disorder. Biopsies from multiple sites showed no malignant findings, and marginal resection with partial curettage was performed. The final diagnosis was locally aggressive fibrous dysplasia, and the restrictive lung disorder improved postoperatively. The natural history of the disease is also unknown. This is the first report in the literature to describe a case in which a lesion exhibited long-term growth over a period of 22 years after reaching adulthood.
INTRODUCTION
Fibrous dysplasia is a tumor-like disorder of the bone caused by abnormal osteogenesis and its lesions generally stop growing when patients reach adulthood. However, malignant transformation should be considered when the growth of tumors or onset of pain is observed in adulthood [1,2]. An extremely rare subtype of benign fibrous dysplasia with cortical bone destruction and soft tissue infiltration can gradually enlarge after bone maturation, despite showing no signs of malignant transformation. To our knowledge, this is the first report in the literature to describe a case in which a lesion exhibited long-term growth after reaching adulthood.
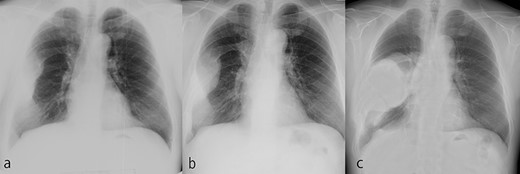
The tumor grew over the course of 22 years after reaching adulthood. (a) Chest X-ray at 50 years old. (b) Chest X-ray at 58 years old. (c) Chest X-ray at 72 years old.
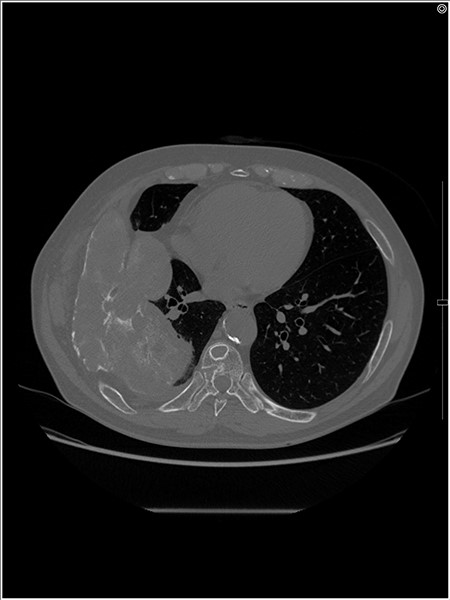
An axial CT image shows the extra-osseous tumor with calcification and cortical destruction of the right seventh rib.
CASE REPORT
A 72-year-old man was referred to our department with a suspected tumor in the right rib. An abnormal shadow in the right chest was found during screening at 50 years of age. The patient was later followed up intermittently by a general physician (Fig. 1a and b). At 72 years of age, he was referred to our department because of a suspected malignant tumor. There were neither subjective symptoms nor abnormal physical findings. A blood test revealed an elevated level for alkaline phosphatase alone at 706 U/L. A pulmonary function test showed a percent vital capacity (%VC) of 78.8%, indicating a mild restrictive impairment. Plain radiography showed a radiopaque tumor measuring 12 × 9 × 18 cm that was connected to the seventh rib in the right thoracic cavity (Fig. 1c). When compared to previous images, the tumor had gradually grown over the preceding 22 years. Computed tomography (CT) showed an extra-osseous tumor-like lesion with calcification, cortical destruction of the right seventh rib and a similar small lesion in the right ninth rib (Fig. 2). Moreover, lesions were also detected in the ninth thoracic vertebral body. Magnetic resonance imaging (MRI) revealed a lower signal intensity inside the lesion compared to the muscle on T1-weighted images and a mixture of low and high intensity on T2-weighted images, while enhancement was observed in the lower intensity area on T2-weighted images (Fig. 3a and b). Bone scintigraphy and positron emission tomography/computed tomography (PET/CT) revealed an increased uptake in the right seventh and ninth ribs and ninth vertebral body, and the right seventh rib showed a high standard uptake value at 7.67 on PET/CT (Fig. 4a and b).
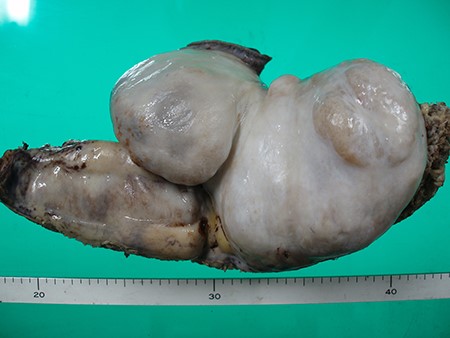
The lesion of the right seventh rib was surgically resected due to enlargement and restrictive lung disease. The right ninth rib was also resected. Because we found that the lesion in the seventh rib had adhered to the lung, which was partially resected. The ninth thoracic vertebral body was not resected. The resected sample was a white solid tumor-like lesion measuring 21 × 8 cm (Fig. 6). No malignant findings were observed despite a complete cleaving and thorough evaluation of the sample. The final diagnosis was locally aggressive fibrous dysplasia. The %VC was increased from 78.8% preoperatively to 95.1% postoperatively and restrictive impairment was improved. The patient had no recurrence for 29 months postoperatively and died of gastric cancer.
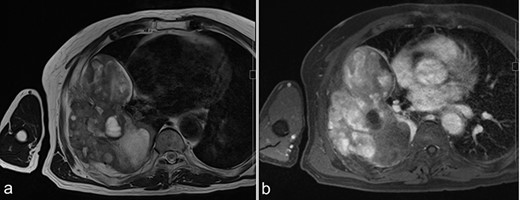
MRI before surgery. (a) T2-weighted MR image shows a mixture of lower and higher intensity areas. (b) An enhanced image shows enhancement in the lower intensity area on T2-weighted images.
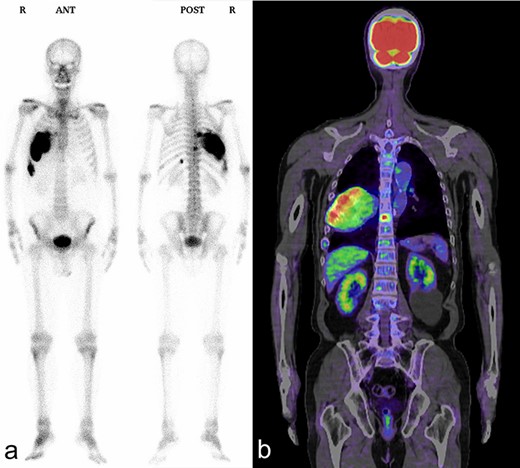
Bone scintigraphy and PET/CT shows an increased uptake in the right seventh and ninth ribs and ninth thoracic vertebral body. (a) Bone scintigraphy; (b) PET/CT.
Because differential diagnoses included malignant transformation from fibrous dysplasia based on the clinical course and imaging, a CT-guided biopsy was performed. Tissue was obtained from various areas with differing contrast effect on MRI. Despite no histological evidence of malignancy, signs of fibrous dysplasia such as irregular osseous trabeculae of immature bone with no osteoblastic rimming were observed. Accounting for possibly insufficient samples, incisional biopsies of the right seventh and ninth ribs were performed. The results showed fibrous dysplasia signs similar to those that were shown by the CT-guided biopsy, and the patient was preoperatively diagnosed as fibrous dysplasia (Fig. 5).
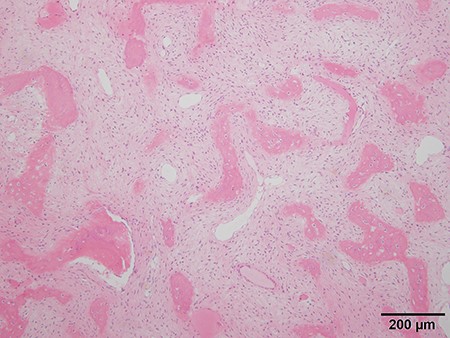
Histologic analysis of the biopsy shows characteristics of fibrous dysplasia with irregular osseous trabeculae of immature bone with no osteoblastic rimming.
DISCUSSION
An enlarged lesion of fibrous dysplasia after bone maturation may suggest malignant transformation. Fibrous dysplasia undergoing malignant transformation often presents with hyper alkaline phosphatemia, and plain radiography or CT often reveal osteolysis or disruption of the cortex [3]. Fluorodeoxyglucose-positron emission tomography/computed tomography (FDG-PET/CT) is reportedly useful for differentiating fibrous dysplasia from metastatic bone tumors and for its early diagnosis of malignant transformation [4]. Our patient presented with hyper alkaline phosphatemia, and CT revealed an extra-osseous mass with cortical bone destruction and calcification. FDG-PET/CT showed strong abnormal accumulation. Furthermore, malignant transformation to low-grade sarcoma was suspected due to the continued growth of the lesion. However, diagnostic imaging for malignant transformation has been reported to cause false-positive results [5, 6]. Therefore, we performed multiple preoperative CT-guided needle biopsies and incision biopsies from multiple sites to confirm that there were no malignant pathological findings before surgery. No malignant findings were found in the final pathological diagnosis of the resected specimen.
Locally aggressive fibrous dysplasia is an unusual subtype of fibrous dysplasia that is characterized by progressive enlargement after bone maturation, cortical bone destruction and soft tissue invasion but without malignant transformation or presence of secondary aneurysmal bone cysts [5]. The etiology and incidence are unknown, but occurrences outside of craniofacial bones are extremely rare, with only 7 reports and 14 cases to date (Table 1). There is currently no established method of treatment. The natural history of the disease is also unknown, and the longest reported follow-up is 9 years. We were able to obtain a long-term follow-up of 22 years, which may provide valuable information for understanding the natural course of locally aggressive fibrous dysplasia. The multi-osseous lesions showed no size changes for the right ninth rib and ninth thoracic vertebral body. A gradual enlargement was observed in the right seventh rib alone and was diagnosed as locally aggressive fibrous dysplasia.
| . | Age (years), sex . | Location . | Follow-up (years) . |
|---|---|---|---|
| Latham et al. [9] | 26, Female | Proximal humerus | 2 |
| Yao et al. [10] | 23, Male | Distal femur | 1 |
| 38, Female | Distal humerus | 0.5 | |
| 47, Male | Distal humerus | 0.25 | |
| Dorfman et al. [11] | 18, Male | Rib | 3 |
| 33, Male | Proximal tibia | 2 | |
| Zídková et al. [12] | Not assessed | Pelvis | Not assessed |
| Hermann and Garcia [13] | 56, Male | Rib | Not assessed |
| Kashima et al. [2] | 60, Male | Rib | 3 |
| 72, Female | Rib | 4 | |
| Muthusamy et al. [5] | 62, Male | Pelvis | 1.5 |
| 70, Female | Pelvis | Not assessed | |
| 65, Male | Rib | 4 | |
| 41, Female | Distal femur | 6 | |
| Present case | 50, Male | Rib | 22 |
| . | Age (years), sex . | Location . | Follow-up (years) . |
|---|---|---|---|
| Latham et al. [9] | 26, Female | Proximal humerus | 2 |
| Yao et al. [10] | 23, Male | Distal femur | 1 |
| 38, Female | Distal humerus | 0.5 | |
| 47, Male | Distal humerus | 0.25 | |
| Dorfman et al. [11] | 18, Male | Rib | 3 |
| 33, Male | Proximal tibia | 2 | |
| Zídková et al. [12] | Not assessed | Pelvis | Not assessed |
| Hermann and Garcia [13] | 56, Male | Rib | Not assessed |
| Kashima et al. [2] | 60, Male | Rib | 3 |
| 72, Female | Rib | 4 | |
| Muthusamy et al. [5] | 62, Male | Pelvis | 1.5 |
| 70, Female | Pelvis | Not assessed | |
| 65, Male | Rib | 4 | |
| 41, Female | Distal femur | 6 | |
| Present case | 50, Male | Rib | 22 |
| . | Age (years), sex . | Location . | Follow-up (years) . |
|---|---|---|---|
| Latham et al. [9] | 26, Female | Proximal humerus | 2 |
| Yao et al. [10] | 23, Male | Distal femur | 1 |
| 38, Female | Distal humerus | 0.5 | |
| 47, Male | Distal humerus | 0.25 | |
| Dorfman et al. [11] | 18, Male | Rib | 3 |
| 33, Male | Proximal tibia | 2 | |
| Zídková et al. [12] | Not assessed | Pelvis | Not assessed |
| Hermann and Garcia [13] | 56, Male | Rib | Not assessed |
| Kashima et al. [2] | 60, Male | Rib | 3 |
| 72, Female | Rib | 4 | |
| Muthusamy et al. [5] | 62, Male | Pelvis | 1.5 |
| 70, Female | Pelvis | Not assessed | |
| 65, Male | Rib | 4 | |
| 41, Female | Distal femur | 6 | |
| Present case | 50, Male | Rib | 22 |
| . | Age (years), sex . | Location . | Follow-up (years) . |
|---|---|---|---|
| Latham et al. [9] | 26, Female | Proximal humerus | 2 |
| Yao et al. [10] | 23, Male | Distal femur | 1 |
| 38, Female | Distal humerus | 0.5 | |
| 47, Male | Distal humerus | 0.25 | |
| Dorfman et al. [11] | 18, Male | Rib | 3 |
| 33, Male | Proximal tibia | 2 | |
| Zídková et al. [12] | Not assessed | Pelvis | Not assessed |
| Hermann and Garcia [13] | 56, Male | Rib | Not assessed |
| Kashima et al. [2] | 60, Male | Rib | 3 |
| 72, Female | Rib | 4 | |
| Muthusamy et al. [5] | 62, Male | Pelvis | 1.5 |
| 70, Female | Pelvis | Not assessed | |
| 65, Male | Rib | 4 | |
| 41, Female | Distal femur | 6 | |
| Present case | 50, Male | Rib | 22 |
This case demonstrated restrictive lung impairment prior to resection, and the resected specimen showed adhesion between the lesion and lung. The resection improved the impairment. Restricted lung impairment due to fibrous dysplasia is a very rare complication. Other complications include respiratory failure, pulmonary arterial hypertension and pulmonary embolism [7,8]. Even for patients without subjective symptoms, resection of the lesion should be considered to alleviate restrictive lung impairment, since continuous growth after adulthood suggests a locally aggressive variant of fibrous dysplasia.
A case of locally aggressive fibrous dysplasia continued to grow for 22 years after reaching adulthood. Locally aggressive fibrous dysplasia requires a careful preoperative diagnosis, as it is sometimes mistaken for malignant tumors and may be overtreated.
Conflict of interest statement
None declared.
Informed consent
Informed consent to publish this article was obtained from the patient and his family.
References
13.



