-
PDF
- Split View
-
Views
-
Cite
Cite
Koujin Miura, Yasushi Adachi, Toshiaki Shirahase, Yoji Nagashima, Kazuki Suemune, Noriko Sakaida, Yorika Nakano, Yasuhiro Sakai, Shigeki Shimizu, Susumu Ikehara, A case of high-grade mucinous tubular and spindle cell carcinoma, Journal of Surgical Case Reports, Volume 2020, Issue 2, February 2020, rjaa014, https://doi.org/10.1093/jscr/rjaa014
Close - Share Icon Share
Abstract
Mucinous tubular and spindle cell carcinoma (MTSCC) is a rare renal cell carcinoma that initially presents as low-grade renal cell carcinoma. However, cases of MTSCC with high-grade histology and poor prognosis have been reported. Here, we report a case of MTSCC with high-grade histological features and metastasis. A 77-year-old woman consulted a hospital following frequent and painful micturition. Computed tomography scan revealed a tumor of the left kidney. First, chemotherapy was performed, with no effects. Therefore, nephrectomy was subsequently performed. Histologically, the tumor showed the features of MTSCC with sarcomatoid component. Metastasis of the tumor into the lymph node was also observed. Although adjuvant chemotherapy was performed after nephrectomy, metastasis to the lungs and bone and local recurrence was observed. The patient is still alive 2 years after nephrectomy with metastasis and recurrence of the tumor. High-grade MTSCC shows a relatively poor prognosis, specifically MTSCC with metastasis upon nephrectomy.
INTRODUCTION
Mucinous tubular and spindle cell carcinoma (MTSCC) in the kidney is a recently described, rare carcinoma. It is mainly observed in middle-aged women, with a man-to-woman ratio of 1:4 [1]. MTSCC is histologically characterized by elongated tubules lined by low cuboidal epithelium and fascicles of spindle cells with stromal mucin [2]. MTSCC initially presents as low-grade renal cell carcinoma, suggesting a good prognosis [1, 3]. However, subsequently, some cases of high-grade MTSCC have been reported [4, 5]. High-grade MTSCC, with Fuhrman nuclear grades 3 and/or 4, metastasizes aggressively. A number of patients diagnosed with high-grade MTSCC have reportedly died. Here, we present a resection case of high-grade MTSCC with lymph node metastasis.
CASE REPORT
A 77-year-old Japanese woman visited a hospital following frequent and painful micturition for 2 weeks, with macrohematuria observed the day prior to consultation. She had hypertension and disk herniation of the lumbar spine. Although her urine showed microhematuria, atypical cells were not observed in her urine in the cytological screening performed at her initial visit to the hospital. Enhanced computed tomography (CT) scan revealed a tumor in the lower part of the left kidney and swelling of regional lymph nodes, suggesting lymph node metastasis of the tumor (Fig. 1). The tumor was mainly observed in the pelvis of the left kidney (Fig. 1A).
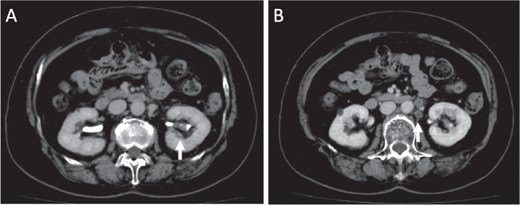
Images of enhanced CT scan. Enhanced CT revealed a 15-mm diameter tumor in the pelvis of the left kidney (A) and swelling of the regional lymph nodes, which showed a low-density area (B).
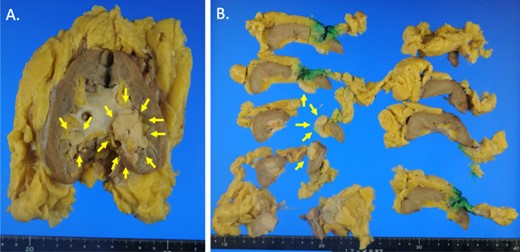
Macroscopic photographs of the tumor. Macroscopic photographs of the coronal section (A) and horizontal section (B) of the kidney. Arrows indicate the tumor.
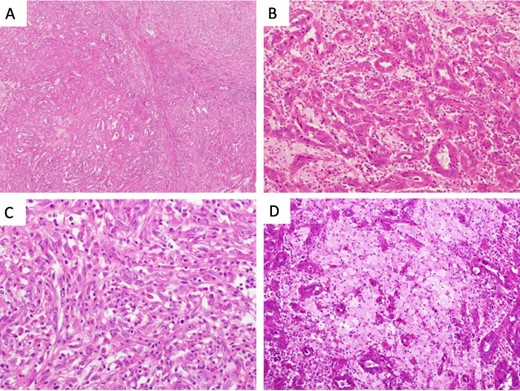
Microscopical photographs of the tumor. Microscopical photographs of the tumor stained by hematoxylin and eosin staining. Original magnifications of the objective lens of A, B, C and D are ×4, ×20, ×20 and ×20, respectively.
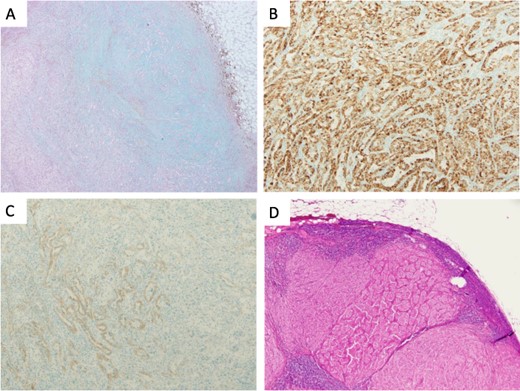
Microscopical photographs of the tumor stained by alcian blue staining and immunohistochemistry of the tumor and the metastatic lesion of the lymph node. The microscopical photographs of tumor with alcian blue staining (A), immunohistochemical staining of AMACR (B) and E-cadherin (C) are shown. A microscopical photograph of lymph node-metastasis of the tumor is also shown (D). Original magnification of the objective lens of A, B, C and D is ×10, ×10, ×10 and ×4, respectively.
Initially, chemotherapy using gemcitabine (1300 mg/body) plus cisplatin (94 mg/day) was started 1 month after her initial visit, resulting in progressive disease. Subsequently, methotrexate, vinblastine, adriamycin and cisplatin therapies (methotrexate, 30–39 mg/body/day; vinblastine, 3.0–3.9 mg/body/day; Adriamycin, 30–39 mg/body/kg; cisplatin, 70–92 mg/body/day) were started. A total of four courses of the therapy were performed, with no effect on the tumor size. Therefore, nephrectomy of her left kidney and dissection of the regional lymph nodes were performed 8 months after her initial visit to the hospital. As shown in Fig. 2, the tumor was observed in the lower part of the pelvis to the parenchyma of the left kidney during macroscopic examination. The tumor size was 35 × 25 × 20 mm, and the color of the tumor was whitish yellow (Fig. 2). Histologically, the tumor consisted of elongated tubular structure and spindle cells with mucinous stroma (Fig. 3A and B). The spindle cell showed enlarged nuclei and prominent nucleoli, suggesting Fuhrman grade 4 (Fig. 3C). Partially, macrophages were prominent in the tumor (Fig. 3D). Alcina blue-positive mucin was also observed in the stroma of the tumor (Fig. 4A). Although the tumor projected to the renal pelvis, the tumor was lined with normal urothelial cells.
Immunohistochemically, the tumor was positive for α-methyl acyl CoA racemase (AMACR), cluster of differentiation (CD) 10, renal cell carcinoma marker (RCC-Ma) and carbonic anhydrase 9 (Fig. 4B and data not shown). The tumor was partially positive for E-cadherin (Fig. 4C). The tumor was negative for cytokeratin (CK) 7, CK20 and CK AE1/AE3 (data not shown) and invaded the vein and metastasized into the regional lymph nodes (11/17) (Fig. 4D). Based on the morphological feature and immunohistochemical staining, the tumor was diagnosed as high-grade MTSCC with regional lymph node metastasis. Administration of pazopanib (600 mg/day) was started 4 months after the operation. Medication was switched from pazopanib to axitinib (10 mg/day) 6 months after the operation. Fifteen months after the operation, metastases in the lung and supraclavicular lymph nodes were observed by CT scan with local recurrence of the tumor. Two years after the operation, bone metastasis of the tumor was also observed. After approximately 2 years and 1 month after nephrectomy, she is still alive but still experiencing tumor metastasis and recurrence.
DISCUSSION
MTSCC is histologically characterized by elongated tubular and spindle cell components with a mucinous stroma. Immunohistochemically, Paner et al. stained 12 cases of MTSCC with anti-AMACR, anti-CK7, anti-CD10, anti-RCC-Ma and anti-c-kit antibodies, with the following immunoreactivities of MTSCC: AMACR, 93%; CK7, 81%; epithelial membrane antigen 95%; RCC-Ma, 7%; CD10, 15%; high molecular weight cytokeratin 15%; and c-kit, 5% [6]. In our case, we diagnosed the tumor as MTSCC based on morphological analyses and positivity for AMACR.
MTSCC was a newly classified rare tumor in renal cell carcinoma. The tumor was first included in the World Health Organization (WHO) classification system in 2004 [1]. Initially, MTSCC was presented as a low-grade renal cell carcinoma. However, some cases of high-grade MTSCC have been reported. Therefore, MTSCC was not limited to a low-grade renal cell carcinoma in the new version of the WHO classification system published in 2016 [7]. In high-grade MTSCC cases, fatal cases with postsurgical metastasis have been reported [4, 5, 8, 9]. In these cases, metastasis of MTSCC in the lymph nodes, pleura and lungs was predominantly observed. We also reported a case of mucin-poor high-grade MTSCC in the kidney with a micropapillary pattern [10]. In this case, the tumor cells demonstrated Fuhrman nuclear grades 2 and 3, and tumor invasion in the lymph vessel and tumor metastasis in the regional lymph node were observed. The patient died due to brain hemorrhage probably induced by brain metastasis of the tumor. In the present case, the tumor has several areas classified as Fuhrman grade 4 with a sarcomatoid component. Several cases of MTSCC with sarcomatoid component have been reported [4, 8]. In these cases, patient experiencing metastasis died, while patients who did not experience metastasis survived without recurrence or metastasis during the follow-up phase. In our case, lymph node metastasis of the tumor was observed on the operated specimen. The patient is alive approximately 2 years after nephrectomy. After operation, metastasis to the lungs and bone and local recurrence were observed although chemotherapy was performed, suggesting that patients with high-grade MTSCC have shown poor prognosis, specifically patients experiencing metastasis when the diagnosis of MTSCC was established.
CONFLICT OF INTEREST STATEMENT
None of the authors have any conflicts of interest to declare relevant to this publication.
FUNDING
No funding was received for this work.



