-
PDF
- Split View
-
Views
-
Cite
Cite
Ryohei Matsushima, Takeshi Mori, Sho Saeki, Hironori Hinokuma, Hidekazu Tanaka, Hiroshi Yokomizo, Long-term follow-up of ciliated muconodular papillary tumor of the lung by computed tomography: a case report, Journal of Surgical Case Reports, Volume 2020, Issue 12, December 2020, rjaa522, https://doi.org/10.1093/jscr/rjaa522
Close - Share Icon Share
Abstract
Ciliated muconodular papillary tumor (CMPT) is an extremely rare pulmonary tumor and the clinical characteristics are still unknown. We report the preoperative long-term clinical course and changes in computed tomography (CT) findings of CMPT. A 60-year-old man underwent lower bilobectomy for squamous cell carcinoma in the right lower lobe 18 years before the surgery for CMPT. Twelve years before the surgery for CMPT, a 4-mm small ground glass nodule arose in the left lower lobe. The nodule gradually grew and became dense over time. Because it became mostly solid with central cavities, the patient underwent wedge resection and the tumor was diagnosed as CMPT. There were no recurrences 20 months after surgery. The preoperative CT findings of CMPT were similar to progressive preinvasive lesion, whereas it followed the benign clinical course. To the best of our knowledge, this is the first report on long-term preoperative follow-up of CMPT.
INTRODUCTION
Ciliated muconodular papillary tumor (CMPT) is a rare lung neoplasm first reported by Ishikawa in 2002 [1]. CMPT has been encountered more frequently with the increase in the availability of the thin-section computed tomography (CT) for lung cancer screening. However, it is often misdiagnosed as adenocarcinoma because the clinical course and time course of CT findings are still unknown [2, 3]. Here, we report the characteristics in CT imaging CMPT followed-up for 12 years.
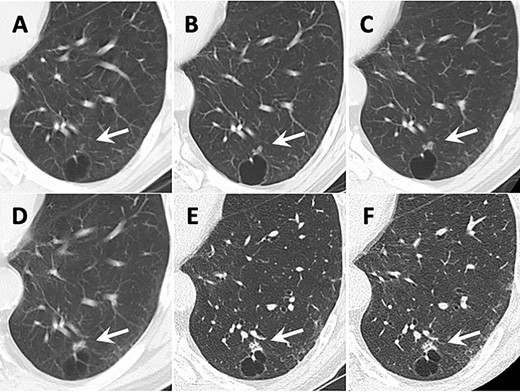
Transition of CT images. Arrows show the tumor. (A) 12 years before surgery. A small ground grass nodule (GGN) adjacent to the cyst wall was identified in the left lower lobe. (B) 9 years before surgery. (C) 6 years before surgery. The GGN showed enlargement. (D) 4 years before surgery. The GGN transformed to the solid lesion gradually. (E) 2 years before surgery. The small intralesional cavities were identified in the nodule. (F) The CT image taken just before surgery.
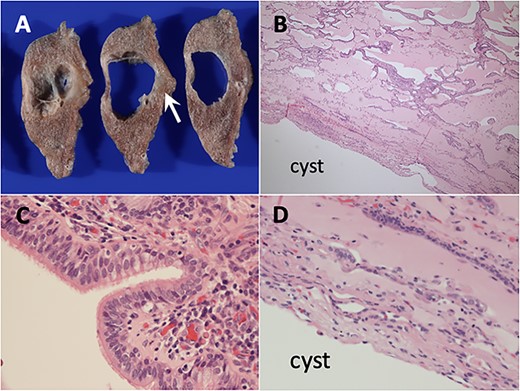
Histopathological findings of ciliated muconodular papillary tumor. (A) Unclearly circumscribed grey–white tumor measuring 10 mm in diameter along the cyst (arrows). (B) Low-magnification view showed peribronchiolar architecture with the alveolar cavities filled with abundant mucus (hematoxylin and eosin stain). (C) High-magnification view showed the tumor constructed of ciliated columnar, mucous and basal cells. (D) The cyst wall did not have any abnormal findings.
Clinical summary of the ciliated muconodular papillary tumor in present case and previous reports
| Author . | Age/sex . | Preoperative CT follow-up duration . | CT findings . | Treatment . | Prognosis . | ||||
|---|---|---|---|---|---|---|---|---|---|
| Tumor growth (increasing size) . | Size (mm) . | Ground glass part . | Solid part . | Central cavity . | |||||
| Ishikawa [1] | 50/F | N/a | N/a | 15 | N/a | N/a | N/a | Lob | 10 y NED |
| Hata [2] | 76/F | 6 mo | − | 7 | − | + | − | Lob | 23 mo NED |
| Chuang [3] | 68/M | 5 mo | − | 7 | − | + | − | WR | 4 y NED |
| Sato [5] | 67/M | 28 mo | + (4 mm) | 9 | + | + | − | WR | 10 mo NED |
| 59/F | 7 mo | + (N/a) | 7 | + | − | + | WR | 18 mo NED | |
| Kon [9] | 80/M | 0 | N/a | 7 | − | + | + | WR | 29 mo NED |
| 67/M | 0 | N/a | 10 | − | + | − | WR | 25 mo NED | |
| 66/M | 0 | N/a | 13 | − | + | + | Lob | 14 mo NED | |
| 73/F | 0 | N/a | 9 | − | + | + | WR | 5 mo NED | |
| 70/F | 0 | N/a | 8 | − | + | + | WR | 48 mo NED | |
| Jin [10] | 59/F | 0 | N/a | 8 | + | + | + | WR | 6 mo NED |
| Harada [12] | 62/M | 0 | N/a | 9 | − | + | − | WR | 2 y NED |
| Present case | 78/M | 12 y | + (6 mm) | 10 | + → - | - → + | - → + | WR | 20 mo NED |
| Author . | Age/sex . | Preoperative CT follow-up duration . | CT findings . | Treatment . | Prognosis . | ||||
|---|---|---|---|---|---|---|---|---|---|
| Tumor growth (increasing size) . | Size (mm) . | Ground glass part . | Solid part . | Central cavity . | |||||
| Ishikawa [1] | 50/F | N/a | N/a | 15 | N/a | N/a | N/a | Lob | 10 y NED |
| Hata [2] | 76/F | 6 mo | − | 7 | − | + | − | Lob | 23 mo NED |
| Chuang [3] | 68/M | 5 mo | − | 7 | − | + | − | WR | 4 y NED |
| Sato [5] | 67/M | 28 mo | + (4 mm) | 9 | + | + | − | WR | 10 mo NED |
| 59/F | 7 mo | + (N/a) | 7 | + | − | + | WR | 18 mo NED | |
| Kon [9] | 80/M | 0 | N/a | 7 | − | + | + | WR | 29 mo NED |
| 67/M | 0 | N/a | 10 | − | + | − | WR | 25 mo NED | |
| 66/M | 0 | N/a | 13 | − | + | + | Lob | 14 mo NED | |
| 73/F | 0 | N/a | 9 | − | + | + | WR | 5 mo NED | |
| 70/F | 0 | N/a | 8 | − | + | + | WR | 48 mo NED | |
| Jin [10] | 59/F | 0 | N/a | 8 | + | + | + | WR | 6 mo NED |
| Harada [12] | 62/M | 0 | N/a | 9 | − | + | − | WR | 2 y NED |
| Present case | 78/M | 12 y | + (6 mm) | 10 | + → - | - → + | - → + | WR | 20 mo NED |
N/a: not available; +: positive result; −: negative result; RUL: right upper lobe; RLL: right lower lobe; LUL: left upper lobe; LLL: left lower lobe; Lob: lobectomy; Seg: segmentectomy; WR: wedge resection; yr: year; mo: month; NED: no evidence of disease.
Clinical summary of the ciliated muconodular papillary tumor in present case and previous reports
| Author . | Age/sex . | Preoperative CT follow-up duration . | CT findings . | Treatment . | Prognosis . | ||||
|---|---|---|---|---|---|---|---|---|---|
| Tumor growth (increasing size) . | Size (mm) . | Ground glass part . | Solid part . | Central cavity . | |||||
| Ishikawa [1] | 50/F | N/a | N/a | 15 | N/a | N/a | N/a | Lob | 10 y NED |
| Hata [2] | 76/F | 6 mo | − | 7 | − | + | − | Lob | 23 mo NED |
| Chuang [3] | 68/M | 5 mo | − | 7 | − | + | − | WR | 4 y NED |
| Sato [5] | 67/M | 28 mo | + (4 mm) | 9 | + | + | − | WR | 10 mo NED |
| 59/F | 7 mo | + (N/a) | 7 | + | − | + | WR | 18 mo NED | |
| Kon [9] | 80/M | 0 | N/a | 7 | − | + | + | WR | 29 mo NED |
| 67/M | 0 | N/a | 10 | − | + | − | WR | 25 mo NED | |
| 66/M | 0 | N/a | 13 | − | + | + | Lob | 14 mo NED | |
| 73/F | 0 | N/a | 9 | − | + | + | WR | 5 mo NED | |
| 70/F | 0 | N/a | 8 | − | + | + | WR | 48 mo NED | |
| Jin [10] | 59/F | 0 | N/a | 8 | + | + | + | WR | 6 mo NED |
| Harada [12] | 62/M | 0 | N/a | 9 | − | + | − | WR | 2 y NED |
| Present case | 78/M | 12 y | + (6 mm) | 10 | + → - | - → + | - → + | WR | 20 mo NED |
| Author . | Age/sex . | Preoperative CT follow-up duration . | CT findings . | Treatment . | Prognosis . | ||||
|---|---|---|---|---|---|---|---|---|---|
| Tumor growth (increasing size) . | Size (mm) . | Ground glass part . | Solid part . | Central cavity . | |||||
| Ishikawa [1] | 50/F | N/a | N/a | 15 | N/a | N/a | N/a | Lob | 10 y NED |
| Hata [2] | 76/F | 6 mo | − | 7 | − | + | − | Lob | 23 mo NED |
| Chuang [3] | 68/M | 5 mo | − | 7 | − | + | − | WR | 4 y NED |
| Sato [5] | 67/M | 28 mo | + (4 mm) | 9 | + | + | − | WR | 10 mo NED |
| 59/F | 7 mo | + (N/a) | 7 | + | − | + | WR | 18 mo NED | |
| Kon [9] | 80/M | 0 | N/a | 7 | − | + | + | WR | 29 mo NED |
| 67/M | 0 | N/a | 10 | − | + | − | WR | 25 mo NED | |
| 66/M | 0 | N/a | 13 | − | + | + | Lob | 14 mo NED | |
| 73/F | 0 | N/a | 9 | − | + | + | WR | 5 mo NED | |
| 70/F | 0 | N/a | 8 | − | + | + | WR | 48 mo NED | |
| Jin [10] | 59/F | 0 | N/a | 8 | + | + | + | WR | 6 mo NED |
| Harada [12] | 62/M | 0 | N/a | 9 | − | + | − | WR | 2 y NED |
| Present case | 78/M | 12 y | + (6 mm) | 10 | + → - | - → + | - → + | WR | 20 mo NED |
N/a: not available; +: positive result; −: negative result; RUL: right upper lobe; RLL: right lower lobe; LUL: left upper lobe; LLL: left lower lobe; Lob: lobectomy; Seg: segmentectomy; WR: wedge resection; yr: year; mo: month; NED: no evidence of disease.
CASE REPORT
A 60-year-old man underwent neoadjuvant chemo-radiotherapy followed by lower bilobectomy for squamous cell carcinoma in the right lower lobe (cT2N2M0 cStageIIIA; 7th edition of the TNM classification) 18 years before the surgery for CMPT. He underwent stereotactic radiotherapy for single brain metastasis 6 months after adjuvant chemotherapy. There was no recurrence of lung cancer at that time.
Twelve years before the surgery for CMPT, a 4-mm small ground grass nodule (GGN) was identified adjacent to a lung cyst wall in the posterior basal segment of the left lower lobe on CT (Fig. 1A). The GGN had gradually enlarged and its density had increased during follow-up (Fig. 1B–D). Two years before the surgery, the nodule measured 9 mm in diameter and the GGN became mostly solid with central cavities inside (Fig. 1E). CT-guided needle biopsy (CTNB) showed no findings of malignancy. Because the nodule kept growing and measured 10 mm (Fig. 1F), re-CTNB was performed and mucinous epithelium growth was identified. The cyst adjacent to the nodule did not show any change during follow-up. The patient had no symptoms, no enlarged lymph nodes and no metastatic lesions during follow-up. The serum levels of tumor markers including carcinoembryonic antigen, cytokeratin-19 fragment and pro-gastrin releasing peptide did not increase.
For the further diagnosis, the patient underwent video-assisted thoracoscopic wedge resection for both diagnostic and therapeutic purposes. Macroscopic examination of the resected lung specimen showed a vaguely-circumscribed grey–white tumor adjacent to cyst measuring 10 mm in diameter (Fig. 2A). Histologically, microscopic examination showed peribronchiolar flat architecture and the alveolar cavities filled with abundant mucus (Fig. 2B). The tumor cells consisted of proliferated epithelium surrounding alveoli including ciliated columnar, mucous and basal cells (Fig. 2C). There was no nuclear atypia, mitosis and necrosis. The normal cells without atypia lay in the cyst wall adjacent to the tumor cells (Fig. 2D). Eventually, the patient was diagnosed as CMPT, especially the distal-type bronchiolar adenoma (BA) that is proposed by Chang [4]. Neither recurrence nor metastasis was detected during the 20-month postoperative follow-up.
DISCUSSION
CMPT is a rare pulmonary neoplasm characterized as a papillary tumor consisting of ciliated columnar cells, mucous cells and basal cells. Because of the pathological difficulty as well as size and location, it is difficult to diagnose CMPT by preoperative biopsy [3, 5]. Therefore, the detailed characteristics of CT imaging and preoperative clinical course are essential for treatment strategy.
The CT features of our present case are as follows: (i) tumor transformed from GGN to solid nodule and (ii) tumor developed slowly. The tumor initially appeared as a small GGN and gradually transformed to a pure solid nodule as it grew. This course of development was similar to early stage lung cancer. Especially in preinvasive lesions, defined in the 2015 fourth edition of the World Health Organization classification, atypical adenomatous hyperplasia (AAH) advances to adenocarcinoma in situ (AIS) and minimally invasive adenocarcinoma (MIA) stepwise. Travis reported that AAH or AIS represented GGN and MIA made solid component inside GGN on CT [6]. There was a difference of growth speed between AAH/AIS and CMPT. Although Lee suggested that the GGN of AAH/AIS basically grew 2.2–2.9 mm per year, the GGN in the present case grew 6 mm in 12 years [7]. These long-term CT findings indicated that CMPT was a slowly progressive disease.
We summarized the clinical features of previous reports and the present case (Table 1). Although the present case had more than 10 years of preoperative CT follow-up, the other cases only had 6–28 months of follow-up. Of the 5 cases with preoperative CT follow-up, 3 cases showed tumor growth. Of the all 13 cases, 11 cases had solid component and 4 cases had ground glass component. The central cavity was described by Kon to represent dilated bronchi or mucus lakes [8]. In the present case, the mucinous lake gradually formed and the central cavity formed progressively as the tumor grew. Other reports mentioned tumor growth and central cavity as characteristics of CMPT but did not refer to how the transformation occurred [2, 8].
The potential malignancy of CMPT has often been discussed. One reason CMPT is considered a malignant neoplasm or precancerous lesion of adenocarcinoma is because driver mutations such as ALK, BRAF V600E and EGFR are found [9]. The immunohistochemical findings of CMPT are also similar to adenocarcinoma [5, 10]. Other reports addressed that the microscopic appearance of CMPT is especially difficult to distinguish from mucinous AIS and well-differentiated papillary adenocarcinoma with cilia formation [2, 3, 10]. Meanwhile, Chang newly proposed the BA that is a family of benign clonal proliferations [4]. He categorized CMPT as a subgroup of BAs. In clinical terms, some literatures suggest CMPT as benign because of its good prognosis [5]. All patients survived with no recurrences after surgery (Table 1). In the present case, there was no rapid tumor growth and no appearance of any metastatic lesions during preoperative follow-up and no recurrence for 20 months after surgery as well.
In conclusion, the preoperative CT findings of CMPT were similar to progressive preinvasive lesion, whereas tumor growth speed was very slow compared with preinvasive lesion and followed benign pre and postoperative clinical course. It is important to keep CMPT in mind when the slowly progressive small pulmonary nodule is recognized on CT. There are limitations to this study as this is a single case report and that we cannot deny the influence of the two CTNB in the formation of CMPT. Further data are required to elucidate clinical characteristics of CMPT.
ACKNOWLEDGMENTS
The authors thank Dr Nagamine at Red Cross Kumamoto Hospital and Dr Nakatani at Yokosuka Kyosai Hospital for pathological diagnosis useful advices. The authors thank MINE editing service for editing the manuscript.
CONSENT FOR PUBLICATION
Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
AVAILABILITY OF DATA AND MATERIALS
All the data are available within the article.
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest to declare.
FUNDING
None.



