-
PDF
- Split View
-
Views
-
Cite
Cite
Genevieve Hattingh, Mariam Ibrahim, Thomas Robinson, Ajay Shah, The effect of hormones on an uncommon breast disorder pseudoangiomatous stromal hyperplasia: a case report and literature review, Journal of Surgical Case Reports, Volume 2020, Issue 12, December 2020, rjaa514, https://doi.org/10.1093/jscr/rjaa514
Close - Share Icon Share
Abstract
Pseudoangiomatous stromal hyperplasia (PASH) is a benign proliferation of the breast, with few cases reported to date. While the etiology of the disease is uncertain, a prevailing theory is that PASH is hormonally responsive, especially in the presence of progesterone. Literature review shows a correlation between PASH development and oral contraceptive pill (OCP) use. We report a case of a 28-year-old autistic female who underwent excision of palpable bilateral breast masses where the histology of the left breast mass identified as PASH. Our patient had a history of multiple medications including OCPs and cytochrome p450 inhibitors that could lead to an increase in progesterone levels. Thus, supporting a theory that medications, in addition to OCPs, may lead to an increased occurrence of PASH in pre-menopausal women.
INTRODUCTION
Pseudoangiomatous stromal hyperplasia (PASH) is an uncommon benign breast condition first described in 1986 [1]. With few reported cases to date, the lesion usually manifests as a palpable lump with similar characteristics to fibroadenomas; however, PASH is also frequently found incidentally on biopsies performed for other reasons [2]. According to the current state of literature, PASH tends to occur most frequently in pre-menopausal women [3], with an additional incidence of 24–47% in men who have gynecomastia [4]. Review of the literature has shown that elevated progesterone levels play a role on PASH. A current working hypothesis links the elevated progesterone levels due to oral contraceptive pills (OCPs), hormone stimulation or other medications to increased incidences of PASH [5].
Diagnosis of PASH is made on core needle biopsy or surgical excision incidentally. On ultrasound (US), PASH typically presents as an oval or round hypoechoic mass or as a heterogeneous mass with cystic areas [4]. On mammography, PASH is commonly described as a well-defined, non-calcified mass with regular borders and margins [4]. Histologically, it is characterized by inter-anastomosing, angulated and slit-like spaces lined by slender spindle cells and surrounded by dense collagenous stroma [3].
PASH may be mistaken for angiosarcoma but can be differentiated based on malignant cytological features and positive immunohistochemical staining of endothelial markers [3]. PASH itself is a benign entity and is not associated with an increased incidence of malignancy. However, PASH can coexist with malignant lesions; suspicious imaging findings should prompt further investigation [1] and close follow-up. Surgical excision may not be indicated in patients diagnosed with PASH on core biopsy. Rather, close observations and serial mammography to assess growth are sufficient [3]. Excision is indicated for tumors with a suspicious radiologic or clinical appearance [6]. Following excision, PASH may recur, and recurrence rates from 5–22% have been reported [1].
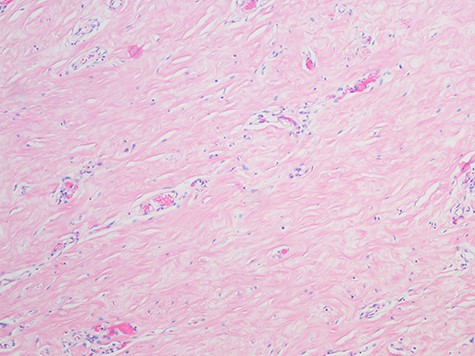
Pseudoangiomatous Stromal Hyperplasia; the slide shows collagenization of interlobular stroma, Myofibroblasts and abundant blood vessels (H&E, magnification x 100).
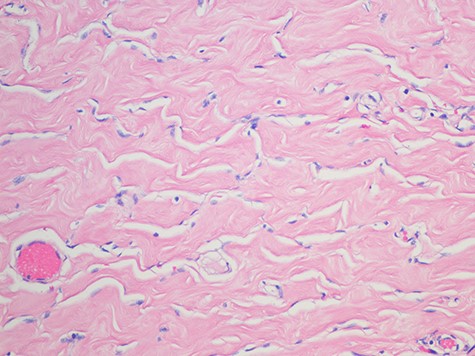
Pseudoangiomatous stromal hyperplasia; showing strands of collagen traverse some spaces and well-formed anastomosing spaces slits outlined by myofibroblasts with uniform, small flat nuclei (H&E magnification x 2).
CASE PRESENTATION
A 28-year-old female was being followed by the breast surgical team since the age of 22 for abnormal breast lesions. She had a complex medical history significant for autism, developmental delay, seizure disorders and encephalitis at age 1. She has been on OCPs for contraception for the past 10 years. Her medications included Risperdal, Topamax, Klonopin and Trileptal. Family history was negative for any breast lesions or ovarian cancers.
She was first seen by the surgical team for left breast fibroadenoma that was removed at age 22. The team continued to follow the patient, and at age 24, a bilateral excisional breast biopsy was conducted. Pathology showed benign mammary tissue with lobular hyperplasia and adenosis. No further intervention was required and the patient was followed with US imaging at regular intervals.
At age 25, she presented with a palpable left and right breast lump. US found a Breast Imaging Reporting and Database System score (BIRADS) 3 lesion showing a right breast hypoechoic lesion at the 4 o’clock position that was approximately 1 cm in size with ill-defined margins. Following discussion with the patient and her family, the decision was made to manage conservatively with regular follow-up imaging and visits to the clinic. Her family assisted to ensure compliance. Six months later, her breast US revealed multiple lesions in both breasts, with an increase in size in the left breast lesion that was now reported as BIRADS 4. Her OCPs were stopped and conservative management continued. Three months later, her repeat US showed a decrease in the size of left breast lesion, which was reported as BIRADS 3.
With continued follow-up, it was noted a year later that she now had palpable breast masses. US again showed the left breast mass had grown from 5.8 cm a year ago to 8.6 cm and an additional right breast lesion. US was read as BIRADS 4. While benign diagnoses of fibroadenomas or fibrocystic changes were the most likely differentials, the decision was made to schedule the patient for surgery due to the size of the masses. The surgical team excised two right and two left breast lesions. The right breast tissue was consistent with fibroadenoma. The left breast tissue showed strands of collagen traversing some spaces and well-formed anastomosing spaces, slits outlined by myofibroblasts with uniform, small flat nuclei consistent with PASH. The patient had no complications from the surgery and was scheduled for a breast US 4 months post-operatively, having missed that appointment. US 1 year post-operatively was read as BIRADS 2. The patient’s mother wanted to ensure yearly follow-ups with the current breast surgeon due to her extensive history of breast lesions.
DISCUSSION
PASH is an infrequent but not a rare major pathologic finding, present in ~6% of benign surgical breast biopsies [7]. It presents in both pre-menopausal and perimenopausal women. To date, the cause remains uncertain; however, a leading hypothesis is that hormonal stimulation plays a role [8], specifically progesterone stimulation. The literature shows a large quantity of progesterone receptor expression in PASH stromal cells, with estrogen receptors expression being more variable [5]. The hypothesis is supported by the fact that, like our patient, there is a higher incidence of PASH in pre-menopausal women [5]. The occurrence of PASH in males further supports the hormonal theory. Pre-menopausal women additionally have a higher usage of OCPs in this population and are at the peak of hormone expression [5].
The patient described in this case lends to credence to the hormonal hypothesis. Not only was she a pre-menopausal woman on OCPs, but her particular drug regimen could have led to elevated progesterone levels. Specifically, our patient was taking Klonopin, Risperidal, Topamax and Trileptal, all medications that interact with cytochrome P (CYPs) 450 enzymes. Multiple hepatic CYPs enzymes are involved in the synthesis and metabolism of estrogen and progesterone. There are at least 17 hepatic CYPs that potentially can participate in the metabolism of steroids. Hepatic CYP enzymes accept a wide range of substrates and multiple CYPs can contribute to the same reaction [9]. Klonopin specifically inhibits CYPs that are directly involved in progesterone metabolization [5].
CONCLUSION
The inhibition of CYPs by Klonopin, resulting in increasing progesterone levels, supports the hypothesis that drugs beyond OCPs may be involved in the development of PASH. We propose future research to investigate the association between PASH and CYP modulators to determine the pathogenesis and risk factors of this uncommon disease.
ACKNOWLEDGEMENTS
We would like to thank Dr Masooma Niazi, MD, Chairman of Pathology at the Bronx Care Health System for the pathology slides.
FUNDING
None.
CONFLICT OF INTEREST STATEMENT
None declared.



