-
PDF
- Split View
-
Views
-
Cite
Cite
Ayse Cetinkaya, Mohamed Zeriouh, Oliver-Joannis Liakopoulos, Stefan Hein, Tamo Siemons, Peter Bramlage, Markus Schönburg, Yeong-Hoon Choi, Manfred Richter, Minimally invasive left atrial appendage (LAA) clip insertion after challenging LAA occluder implantation to minimize the risk of stroke, Journal of Surgical Case Reports, Volume 2020, Issue 11, November 2020, rjaa432, https://doi.org/10.1093/jscr/rjaa432
Close - Share Icon Share
Abstract
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia, requiring lifelong anticoagulation or interventional, transseptal left atrial appendage (LAA) occluder implantation to minimize stroke risk. Incomplete LAA closure post implantation is a frequent observation. Incomplete LAA occlusion after transseptal occluder implantation necessitates anticoagulation in cases of persistent AF to minimze risk of embolism and/or apoplexy. Patients with contraindications to lifelong anticoagulation therapy are challenging to treat and alternative options are needed. We present a case of a patient with persistent AF who underwent frustraneous LAA occluder implantation. The patient’s anatomy necessitated surgical closure of the LAA, which was accomplished with an LAA clip 4 weeks after implantation. The patient was discharged in excellent clinical status 5 days after the surgery. No further complications were observed within the following year.
INTRODUCTION
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia and a major risk factor for stroke [1–3]. Gold-standard stroke prevention is lifelong anticoagulation, which is not suitable for all patients [3, 4]. Overall, 90% and 57% of thrombi found in non-valvular and valvular AF patients, respectively, are in the LAA, making it a target for stroke prevention [3]. LAA occlusion devices are a mechanical alternative to oral anticoagulation [4, 5]. Cases of challenging primary transseptal LAA occluder implantations have been reported, particularly in patients with certain types of LAA anatomy [6], which can result in small peri-device leaks and increase thromboembolism risk [7]. We report on a patient with frustraneous LAA occluder implantation and complete LAA closure.
CASE REPORT
Patient history
A 63-year-old patient presented with persistent AF. On hospital admission, the patient was in good condition, with a body mass index of 24 kg/m2. Patient history revealed that 15 years previously they suffered a cerebral haemorrhage and recovered without evidence of residual lesions. A routine examination 3 years ago, identified persistent AF with a CHADS-VASc score of 2 (hypertension +1, stroke +1). Ejection fraction was normal (55%). The patient was on 2.5 mg bisoprolol twice daily for rate control. Treatment (20 mg rivaroxaban pod) was initiated. The patient suffered recurrent cerebral bleeding ~ 3 months ago; anticoagulant treatment was stopped.
LAA occluder implantation
A treatment review for AF was undertaken, but no further action such as MAZE, pulmonary vein isolation, electrophysiology intervention or rhythm control were considered viable strategies. An LAA occluder implantation (Amplatzer Amulet LAA Occluder, Abbott) was carried out under transoesophageal echocardiography (TEE)-guidance and general anaesthesia (Figs 1 and 2). The left atrium was accessed via the right femoral vein (7F 8.5F catheter). The transseptal puncture with an SL1 catheter was pressure-controlled and TEE-guided posterior–inferior, straight to the optimal position. A 6F pigtail catheter was inserted for LAA angiography in right anterior oblique 30°, cranial 20° and caudal 0°.
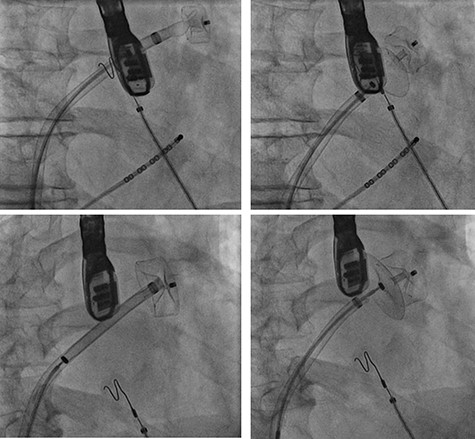
Cardiac catheterization: LAA occluder implantation; interventional LAA occluder implantation via the right femoral vein and septal puncture under radioscopy and transoesophageal echocardiography (TEE) control.
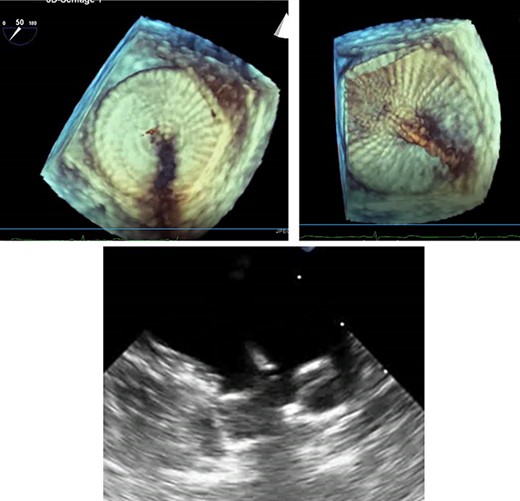
TEE for interventional LAA occluder implantation; TEE controlled, unsuccessful LAA occluder implantation due to difficult LAA morphology.
The patient’s anatomy showed a large funnel-shaped ostium, with narrow neck and shallow depth and chicken-wing morphology with 90° anterior/frontal angulation. TEE measurements showed a landing zone diameter of ~12–13 mm. A 16 mm occluder was selected. The TorqueVue lock 12F was exchanged via a stiff wire and positioned in the left superior pulmonary vein. No satisfactory position was achieved. For the proximal part, shortly after the ostium with a maximum depth of 12 mm the occluder was too small and exchanged for a 20 mm occluder. This could not be securely positioned; the corpus dislocated in the Timed Up and Go test because of limited contact with the LAA surface. A new transseptal puncture was performed in a more posterior and superior position, the CS catheter removed, and the 7F sheath changed to the SL1. The Amplatzer Amulet 16/20/25 mm LAA occluder implantation remained challenging due to the unusual anatomy with an atypical position of the auricular appendage. Finally the procedure was aborted in favour of a LAA clip.
During the procedure and subsequent telemetric monitoring, recurrent bradycardia and sinoatrial block with pauses of ~5 s was noticed. Asymptomatic left atrial tachycardia with heart rate ~150/min occurred. The patient was diagnosed with sick sinus syndrome, but remained asymptomatic during arrhythmias. The presence of tachycardia-bradycardia syndrome was an indication for pacemaker implantation and a DDD pacemaker (Assurity DR MRI, Abbott) was implanted 1 week later and showed appropriate aggregate function.
LAA clipping
Surgical implantation of an LAA clip using a minimally invasive technique was discussed. Fondaparinux (2.5 mg once daily) was initiated due to CHA2DS2-VASc score of 2 and increased HAS-BLED score after two bleeding episodes. After ~4 weeks, the patient was admitted to our department.
A cardiac computed tomography excluded coronary heart disease and showed the LAA was raised anteriorly at the level of the left upper pulmonary vein and had a chicken-wing shape, with an anterior curvature to the right (Fig. 3). Ectasia of the ascending aorta (41 × 41 mm at pulmonary artery level) was detected.
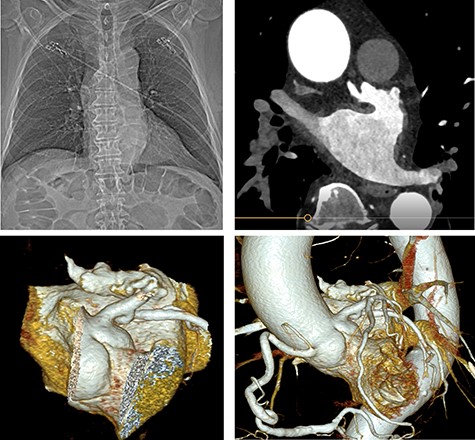
Preoperative cardiac computed tomography (CT) ‘Chicken wing’ morphology of the LAA; cardio CT for presentation of the special (Chicken wing morphology) of the LAA and for exclusion of coronary heart disease before surgical LAA clip implantation.
An LAA clip was implanted, under general anaesthesia with a double lumen tube, via a left mini-thoracotomy without a heart-lung machine. Briefly, a 7 cm skin incision was made laterally above the left mamilla (Fig. 4). Preparation of the subcutis and pectoralis muscle in the fourth intercostal space exposed the LAA. The LAA clip (AtriCure 40 mm) was placed over the LAA to its base. Transoesophageal echocardiography was used to complete LAA closure, the clip was closed and holding device removed (Fig. 5). The patient received 2.5 mg fondaparinux once daily to prevent thrombosis formation; once mobile anticoagulation was stopped. Five days post intervention, the patient was discharged in good clinical condition. Wound healing was uneventful at 10 days. After 1 year without anticoagulation, no further complications were observed.
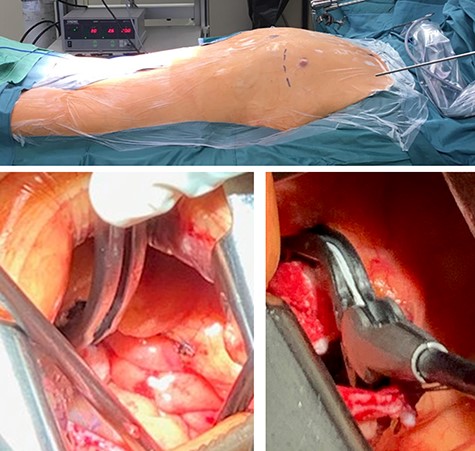
Intraoperative photographs: set-up and LAA clip; intraoperative setup and intra-thoracic images of the LAA clip positioning/implantation.
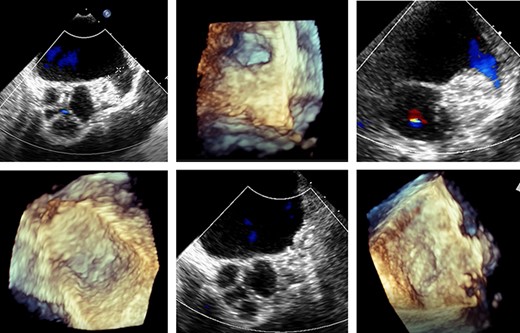
Transoesophageal echocardiography control of the LAA clip in correct position at the LAA base with display of the complete LAA closure.
DISCUSSION
AF is a risk factor for stroke and necessitates either pharmacological intervention or mechanical intervention with implantation of an LAA occluder device [3]. Interventional LAA closure via transseptal puncture for LAA occluder implantation is a standard therapy, but is challenging in patients with certain morphologies/large LAA [6]. The safety and effectiveness of LAA occluder implantation in patients with non-valvular AF is established [4, 8], but can be associated with incomplete LAA closure [7, 9]. LAA anatomy can complicate implantation, potentially contributing to incomplete occlusion/increasing thrombosis risk [10]. We show that minimally invasive LAA clip implantation via left lateral mini thoracotomy is safe and effective for LAA closure.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
None.



