-
PDF
- Split View
-
Views
-
Cite
Cite
Leilei Shen, Jixing Lin, Zhipeng Ren, Bailin Wang, Kai Zhao, Yunlong Lu, Fulin Wang, Lianbin Zhan, Ciliated muconodular papillary tumor of the lung: report of two cases and review of the literature, Journal of Surgical Case Reports, Volume 2019, Issue 8, August 2019, rjz247, https://doi.org/10.1093/jscr/rjz247
Close - Share Icon Share
Abstract
Ciliated muconodular papillary tumor (CMPT) is a peripheral non-endobronchial lung nodule, consisting of ciliated columnar cells and goblet cells with basaloid cell proliferation. Only about 50 cases confirmed by surgery have been reported in English literature worldwide. We present two surgical cases of CMPT in this report. Two patients presented with abnormal computed tomography findings but no obvious symptoms. The first patient’s intraoperative frozen examination was unable to distinguish benignity from malignancy, and he received lobectomy. The other patient’s intraoperative frozen examination indicated adenocarcinoma, but she received wedge resection for her refusal to lobectomy. The two patients’ postoperative pathological analysis finally confirmed the diagnosis of CMPT. We believe that our cases may be essential for pathologists and surgeons to improve their understanding.
INTRODUCTION
Ciliated muconodular papillary tumor (CMPT) is an extremely rare peripheral pulmonary tumor first described in 2002 by Ishikawa [1], consisting of ciliated columnar cells, mucous cells and basal cell proliferation. More and more details of the clinicopathological characteristics have been defined with increasing cases confirmed by surgery recently [2–5]. Recent studies also have reported the presence of driver gene mutations [5]. But it is still difficult for pathologists to differentiate benignity from malignancy, especially on frozen sections and small biopsies. CMPT can easily be misdiagnosed as adenocarcinoma and therefore be overtreated. Here, we report two surgical cases of CMPT.
CASE REPORT
Patient 1
A 58-year-old man was admitted to our hospital due to abnormal chest shadow without any symptoms. Chest computed tomography (CT) revealed an 11-mm peripheral nodule in the right S10 segment (Fig. 1A). He received video-assisted thoracoscopic surgery (VATS), and wedge resection was carried out. The intraoperative frozen examination indicated papillary carcinoma and was unable to distinguish benignity from malignancy. Afterward, a right lower lobectomy was performed.
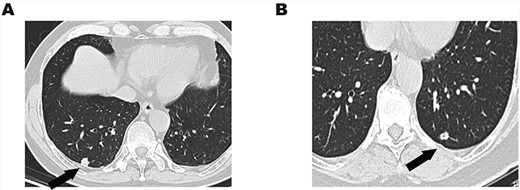
(A) Chest CT showed an 11-mm solitary nodule with central cavity in the right S10 segment. (B) An 8.5-mm solitary nodule with central cavity in the left S10 segment.
Postoperative pathological analysis showed that the tumor cell consisted of proliferated epithelium with adenoid and papillary structures, including ciliated columnar, mucous and basal cells. Tumor cells showed no atypia, mitosis or necrosis (Fig. 2A). The immunohistochemical profile of the ciliated columnar and mucous cells showed that cytokeratin (CK) 7 was positive; thyroid transcription factor 1 (TTF-1) was locally positive. P63 was positive in basal cells (Fig. 3 Case 1). A low Ki-67 proliferating index can be seen in CMPT.
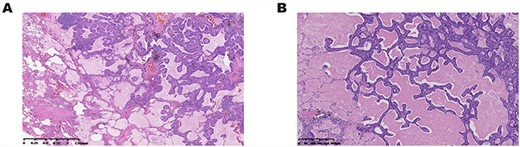
(A) Postoperative pathological analysis showed ciliated columnar cells were coated on the surface of adenoid or papillary structures, and basal cells were located in the outer layer. (B) Histopathological findings showed ciliated columnar cells with mucous lakes.
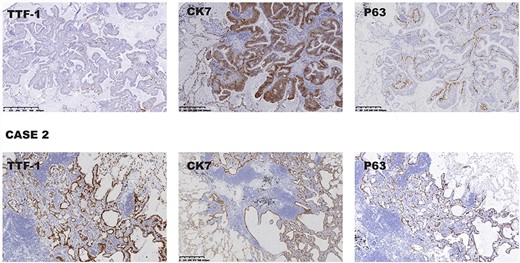
(Case 1) The immunohistochemical profile of the ciliated columnar cells and mucous cells showed that TTF-1, CK7 and P63 were positive. (Case 2) Representative photographs showing positive immunohistochemistry results for TTF-1, CK7 and P63.
Finally, this case was pathologically diagnosed as CMPT. Postoperative genetic analysis showed that BRAF V600E mutation, epidermal growth factor receptor (EGFR) mutation and Kirsten rat sarcoma viral oncogene (KRAS) mutation were all negative.
Patient 2
A 64-year-old woman was admitted to our hospital due to a gradually enlarged nodule in the left S10 segment. The nodule was about 5 mm when it was first found in 2007. It became 8.5 mm in 2019 (Fig. 1B). She insisted on receiving wedge resection instead of lobectomy even if the nodule was malignant. The intraoperative frozen examination indicated adenocarcinoma, and there is no further operation for her.
Postoperative pathological analysis confirmed the diagnosis of CMPT, including the histopathological findings (Fig. 2B) and immunohistochemical profile (Fig. 3 Case 2). Genetic analysis showed EGFR mutation was negative.
DISCUSSION
CMPT is a peripheral non-endobronchial lung nodule arising in older asymptomatic patients, ranging in size from 4 to 45 mm. But we found a teenage-girl case [3]. The tumor could occur in either sex, without any correlation with smoking history. The lesion could be partially solid, solid or appear as ground-glass nodules [2]. Central cavitation could be seen in quarter of reported cases [2–4] and in our report. A number of reported cases and ours with follow-up CT findings before surgical excision have shown size enlargement [5]. Benignity could not be confirmed on the basis of CT findings alone, and it is often considered as adenocarcinoma or metastasis due to the irregular shape and peripheral location. Miyai et al. [6] reported the clinical use of preoperative positron emission tomography–CT scanning without any positive findings. Non-operative biopsy, including bronchoscopy and percutaneous puncture, is also difficult to perform. Therefore, patients would choose VATS to diagnose and treat the nodule.
Generally, the intraoperative frozen section is challenging to differentiate benignity from malignancy because of the low quality of the specimens [7]. Mikubo et al. [8] suggested that touch imprint smear cytology is useful for obtaining an accurate diagnosis in frozen sections. Postoperative pathological examination could confirm a definite diagnosis. CMPT should be differentiated from several more common tumors, including adenocarcinoma, mucoepidermoid carcinoma and papilloma. The key to diagnosing CMPT is to identify [9]: (i) tripartite cellular elements including ciliated columnar cells, mucinous cells and basal cells; (ii) papillary tumors accompanied with mucous production and (iii) tumor cells without atypia, mitosis or necrosis.
Immunohistochemical studies presented in previous reports [2–4] had shown positive immunoreactivity for CK 7, as well as focal-positive results for carcinoembryonic antigen and TTF-1. There is variable expression of mucin (MUC) 1 and MUC5AC, while CK 20 and napsin A expression are negative. And low Ki-67 proliferating index was seen in all tumors, which indicated their benign nature. But Miyai et al. [6] reported a case of the malignant transformation of basal tumor cell components, which indicated the possibility of CMPT’s low-grade malignancy. Molecular studies showed several identified gene mutations, including BRAF mutations, EGFR exon 19 deletions, ALK gene rearrangements and so on [3, 5]. These reports supported the concept that CMPT is a neoplastic disease as opposed to a reactive or metaplastic lesion [10]. Because mutations in oncogenes such as EGFR and KRAS have been connected with the pathogenesis of lung adenocarcinoma, some researchers considered that the presence of EGFR and KRAS mutations in CMPT may be a precancerous lesion or a low-grade malignant tumor of mucinous adenocarcinoma [3].
To date, no consensus has been reached on the benignity or malignancy of CMPT, most researchers would consider it as benign or low-grade malignant tumor. Therefore, local resection of the lung is the optimal approach. When CMPT is misdiagnosed as adenocarcinoma in intraoperative frozen examinations or malignancy cannot be ruled out, we should choose the standard surgical modality for lung cancer, like our first case did. Regardless of the type of surgical intervention, no recurrences or metastasis has been reported so far [10].
In summary, CMPT is a rare peripheral pulmonary tumor found incidentally by chest CT. The presence of ciliated, mucous and basal cells and lack of mitotic figures and necrosis may help diagnose. It is likely benign, and local resection may be sufficient. The accumulation of similar cases will help pathologists to clarify its exact disease nature and surgeons to improve their understanding of such tumor, so that misdiagnosis and overtreatment can be avoided.
Funding
No funding provided for this report.
Conflict of Interest
None declared.



