-
PDF
- Split View
-
Views
-
Cite
Cite
Geng Ju Tuang, Farah Dayana Zahedi, Izzah Akashah, Jennifer Peak Hui Lee, Zainal Azmi Zainal Abidin, An unusual presentation of sphenoid Candida fungal ball: a case report, Journal of Surgical Case Reports, Volume 2019, Issue 8, August 2019, rjz240, https://doi.org/10.1093/jscr/rjz240
Close - Share Icon Share
Abstract
The clinical presentation of a sphenoid fungal ball (FB) is often non-specific and tends to be overlooked, particularly in hosts with an intact immune status. Rarely, potentially life-threatening complications may arise, owning its anatomical characteristics with contiguous structures. Herein, we present an unusual case of sphenoid FB complicated with orbital apex syndrome in an immunocompetent patient. The diagnosis dilemma and subsequent management are further discussed.
Introduction
Fungal infection of the paranasal sinus encompasses a broad spectrum of diseases ranging from indolent colonization to aggressive and potentially life-threatening state, of which its clinical manifestation is generally determined by the host immune status [1]. Based on the histopathological evidence of hyphae invasion into its residing mucosa layer, fungal rhinosinusitis is broadly categorized into two groups [1, 2]. The invasive form is comprised of acute fulminant, granulomatous, and chronic invasive fungal sinusitis. They tend to occur in hosts with impaired immunity and frequently associated with high morbidity and mortality rate. On the contrary, the characteristics of a non-invasive form of fungal rhinosinusitis are protracted and unobtrusive, often observed in immunocompetent patients. These include fungal ball (FB) and allergic fungal rhinosinusitis [1, 2].
Orbital apex or Tolosa-Hunt syndrome is a constellation of cranial neuropathies involving optic, oculomotor, trochlear, abducens, and ophthalmic distribution of trigeminal nerves [3]. It is regarded as a neuro-ophthalmic emergency, therefore requires instant recognition and treatment to avert disastrous consequences. Orbital apex syndrome as a complication from non-invasive fungal sinusitis is a rare entity. The prognosis is favourable with timely intervention.
Case Report
A 40-year-old Indian gentleman with no known comorbid was referred to the otorhinolaryngology department for assessment of his nasal cavity and nasopharynx, following an incidental finding of a suspicious lesion within the left sphenoid sinus based on computerized tomography (CT). He presented with 2 weeks history of intermittent unilateral headache not relieved by conventional analgesics. It was described as throbbing in nature located at the left frontotemporal region. The pain was not precipitated by bending forward or coughing. He also did not complain of photophobia or autonomic symptoms. Unfortunately, his symptoms worsened with a new onset of double vision of 1-week duration. There was an absent history of fever, ear, nose, and throat symptoms. Further history did not suggest any possible risk factors for head and neck malignancy as well as immunodeficiency.
Clinically, he was well built with stable vital signs and a full Glasgow Coma Scale (GCS). The ophthalmological assessment demonstrated restrictions in all left extraocular muscle’s movement (Fig. 1). The visual acuity of both eyes was not affected. However, relative afferent pupillary defect of his left eye was positive, evidenced by red desaturation of 50%. The anterior chamber, intraocular pressure, and fundi were unremarkable. In addition to that, the left corneal sensation was reduced, indicative of the fifth cranial nerve involvement. The rest of the cranial nerve’s functions were intact. Apart from oedematous mucosa overlying the left spheno-ehtmoidal recess, endoscopic evaluation of the nasal cavity revealed neither tumour growth nor purulent discharge from the paranasal sinus and nasopharynx. Assessment of the head and neck region was otherwise normal.
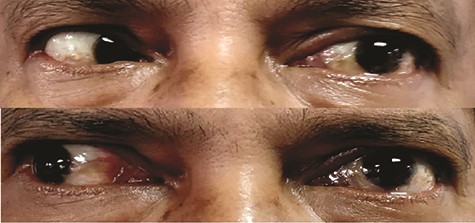
Presence of restricted left extraocular muscles movement on temporal gaze (TOP) and nasal gaze (BOTTOM).
CT unveiled calcified densities within areas of hypoattenuation in the left sphenoid sinus. No marked bony destruction was observed (Fig. 2). A subsequent magnetic resonance imaging (MRI) was carried out to show hypodensity on T1 weighted post-contrast, and flow void in T2 weighted images within the same sinus (Figs. 3 and 4). Following informed consent, an emergency endoscopic left trans-ethmoidal sphenoidotomy was performed under general anaesthesia. A dense, darkened clay-like mass surrounded with the mucopurulent discharge was noticed within the left sphenoid sinus. The mucosa appeared inflamed and oedematous without clinical evidence of frank necrosis. The debris was utterly removed with a curette and sent for evaluation. The left sphenoid sinus was widened and thoroughly rinsed.
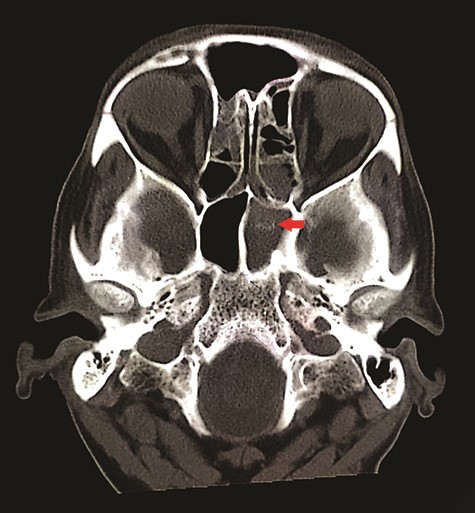
Axial view of non-contrasted CT bone window showed ‘double density’ sign within the left sphenoid sinus (red arrow); there was hyper attenuation observed in the centre of the sinus with surrounding mucosal thickening, suggestive of fungal concentration.
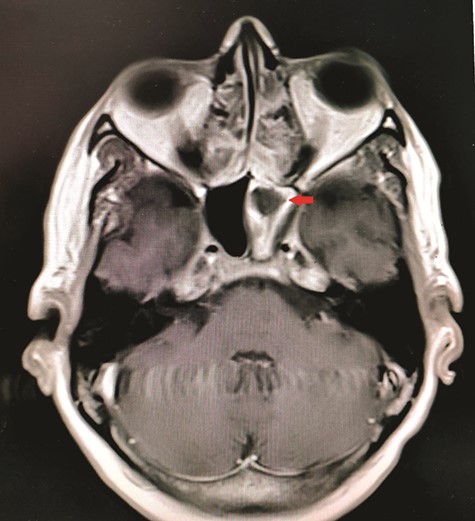
Axial view of MRI T1 weighted post-contrast showed hypodensity within the left sphenoid sinus with surrounding inflammation (red arrow).
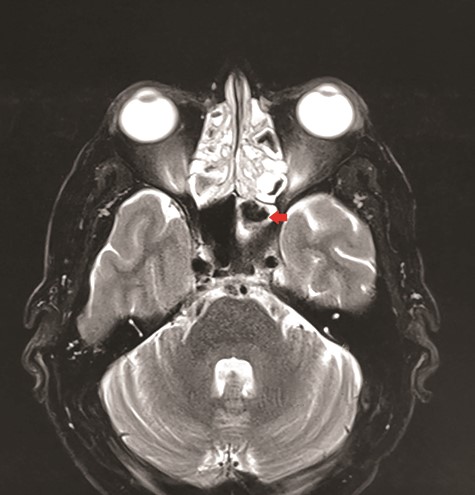
Axial view of MRI T2 weighted revealed flow void signal within the left sphenoid sinus.
Histopathological examination showed Candida species without evidence of mucosal invasion (Figs. 5 and 6) Intravenous ceftriaxone was commenced for a total duration of 14 days. His symptoms showed significant improvement 1 week after the operation with complete resolution of the headache. His left extraocular muscle functions were slowly restored at 4 weeks post-operation (Fig. 7).
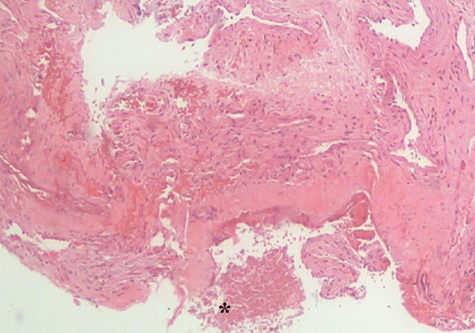
Histopathological examination revealed fungal body (*). No evidence of mucosa invasion was observed.
Discussion
Fungal infection of the paranasal sinus was first reported by Zarniko in the 18th centuries [4]. Owing to the remarkable advancement in science and technology, which permits a far superior detection and evaluation of the disease, there is a strong emergence of fungal sinusitis for the past two decades [4]. Mycetoma, aspergilloma, chronic non-invasive granuloma, and FB are the interchangeable terms used to describe an extra mucosal conglomeration of intertwined fungal hyphae or pseudohyphae, without histopathological evidence of invasion [4, 5]. When the paranasal cavity region is involved, the commonest affected sinus is the maxillary sinus followed by sphenoid sinus [2, 4]. It generally occurs in immunocompetent hosts as unilateral disease, even though bilateral involvement has been reported [2, 6]. FB of the paranasal sinus is reported to have a female predominance, commonly affecting the middle and elderly age group [2, 4, 6]. It is hypothesized that an insufficient mucociliary clearance system in which fungal microorganism are not cleared from one of the sinuses distal to the obstruction as a result from trauma, previous nasal surgery or radiotherapy, leading to the development of FB [7].
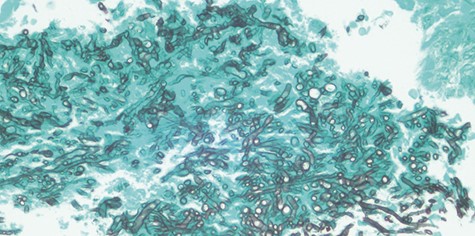
Gomori’s methenamine silver (GMS) stain of Candida species evidenced by its septate hyphae with characteristic dichotomous branching at an angle of approximately 450.
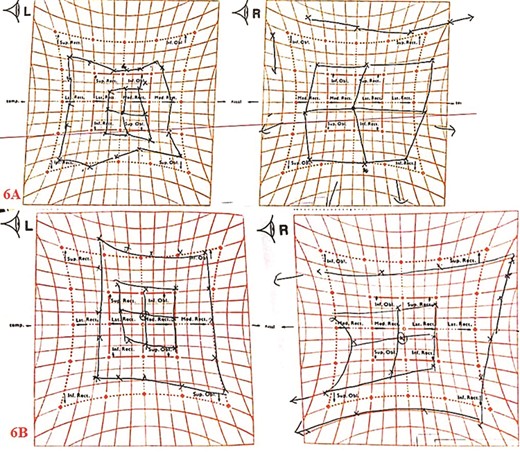
Hess screen chart showed overall improvement of the left extraocular muscle movements; pre-operation (6A); 4-weeks post-operation (6B).
The clinical presentation of a FB differs in accordance with sinus involved. The nasal symptoms such as cacosmia, mucopurulent discharge, and congestion are predominantly seen in maxillary and ethmoid FB. The additional supporting sign includes facial tenderness on the affected side [2]. On the other hand, the manifestation of sphenoid FB is non-specific and tends to mimic other common pathologies such as tension headache or migraine, thereby clouding the diagnosis making [8]. These vague clinical manifestations can be attributed to the deep-seated anatomical location of sphenoid sinus, which is also intimately associated with vital structures such as the brain, cranial nerves, and major vessels. Cranial nerves involvement in sinonasal FB is rarely reported [3]. Our patient initially presented with a short history of intermittent unilateral headache over the frontotemporal region without any nasal complains. Despite a further deterioration in his condition with new onset of ophthalmoplegia, the diagnosis was ambiguous judging from his normal endoscopic finding, radio imaging results, and most importantly, his intact immune status. Nevertheless, an urgent endoscopic sphenoidotomy was performed for diagnostic and curative purpose. Final histopathological result confirmed non-invasive Candida FB. The novelty of the underlying mechanisms for the cranial nerves’ involvement in non-invasive FB remains under debate. In our presenting case, the progressive mucosa inflammation, along with secondary bacterial infection beyond the sphenoid sinus, could be the contributing factors. The extension of the inflammatory process from sphenoid sinus to the adjacent nerve sheath was postulated to be the culprit for cranial neuropathies [9, 10]. In his retrospective review of thirteen patients with an isolated sphenoid lesion, Lee et al. [9] hypothesized that pressure ischaemia, as well as vascular infarction resulting from thrombophlebitis, led to cranial neuritis. Karkas et al. [10] stated that orbital and cranial complications might ensue through haematological seedling of bacterial superinfection in cases of sphenoid FB.
Radiological modalities are deemed invaluable in the management of sphenoid FB [8]. Non-contrasted CT aids to delineate the bony structures of the paranasal sinus as well as to rule out other possible skull base pathologies. FB typically shows ‘hyperdense spot’ within a hypo attenuated paranasal sinus lining and is often associated with sclerosis of the adjacent bone [6, 7]. In contrast, acute invasive fungal sinusitis may demonstrate unilateral sinus opacification with soft tissue thickening and aggressive bony destruction [7]. MRI is useful to evaluate the surrounding soft tissue structures when involved. FB may present as hypointense in both T1 and T2-weighted owing to the absence of free water. Heavy metal depositions within the FB may reflect as a signal void in T2- weighted image [7]. Conventional dogman in the cure of a FB dictates a complete surgical removal of the lesion without the warrant of peri or post-operative antifungal treatment [1, 8]. Prognosis is generally good with a resolution of symptoms [2, 8].
Conclusion
In summary, the clinical manifestation of sphenoid FB is vague and should be included as one of the differential diagnosis in patients presented with unilateral headache. A diligent history taking and clinical evaluation are utmost important. Radiological assessment of the paranasal sinus is invaluable to facilitate the management. Given the proximity of its anatomical locations to the surrounding vital structures, early surgical intervention should be carried out in symptomatic patients to avert possible disastrous outcome.
Consent
Written consent was obtained from the patient for publication of this case report and accompanying image.
Conflict of interest statement
None declared.



