-
PDF
- Split View
-
Views
-
Cite
Cite
Alyson S Cunningham, Ayesha S Siddique, Saverio Ligato, Paul V Vignati, A large inflammatory fibroid polyp of the rectum removed by transanal excision, Journal of Surgical Case Reports, Volume 2019, Issue 6, June 2019, rjz164, https://doi.org/10.1093/jscr/rjz164
Close - Share Icon Share
Abstract
Inflammatory fibroid polyps (IFP) are rare benign lesions arising from the submucosa of the gastrointestinal tract, most commonly found in the stomach and small intestine. IFPs are very rarely found in the rectum, with only a few reported cases, and their presentation is quite varied. The patient is a 53 year old male who underwent routine screening colonoscopy, during which a lobular mass of the proximal rectum was discovered. The patient subsequently underwent an endoscopic ultrasound (EUS) with fine needle aspiration (FNA) biopsy. Pathology displayed scant spindle cells with benign glandular epithelium suspicious for a spindle cell neoplasm. The mass was excised transanally. The morphological and immunohistochemical findings supported the diagnosis of inflammatory fibroid polyp. Although this is not a malignant tumor, the treatment and surveillance guidelines have not been determined and there is no standard of care.
INTRODUCTION
Inflammatory fibroid polyps (IFP) are rare benign lesions arising from the submucosa of the gastrointestinal tract, most commonly found in the stomach and small intestine. IFPs are very rarely found in the rectum, with only a few reported cases [1–6], and their presentation is quite varied. They may present at any age, though usually are discovered in adults. The diagnosis of an IFP is contingent upon the pathology. Presently, little data exits regarding the etiology, treatment recommendations, and recurrence rate.
CASE REPORT
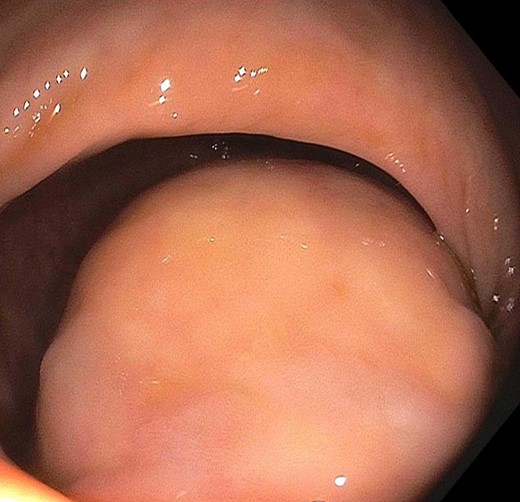
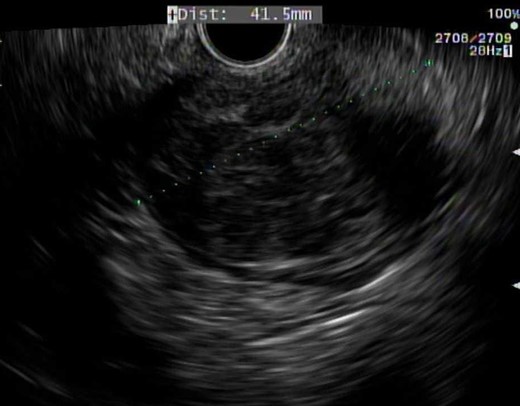
The patient is a 53-year-old male with past medical history significant for type II diabetes mellitus and hypercholesterolemia who underwent routine screening colonoscopy, during which a lobular mass of the proximal rectum was discovered (Fig. 1). The patient subsequently underwent an endoscopic ultrasound (EUS) with fine needle aspiration (FNA) biopsy (Fig. 2). Pathology displayed scant spindle cells with benign glandular epithelium suspicious for a spindle cell neoplasm. Accordingly, the patient was evaluated by a colorectal surgeon for further plan of care. A firm palpable lesion was appreciated on digital rectal exam. Various surgical approaches including laparoscopic low anterior resection as well as transanal excision were discussed with emphasis on complete excision due to the possibility of malignancy. The patient was taken to the operating room and an approximately 3 to 4 cm mass of the mid-rectum was appreciated; as the mass was found to be mobile, it was excised transanally by means of a stapler. Intra-operative pathological evaluation deemed the mass likely to be a low-grade spindle neoplasm, raising the possibility of a schwannoma or gastrointestinal stromal tumor (GIST). The procedure was well tolerated and the patient experienced full continence postoperatively.
On excision, the lesion was grossly a 4.2 cm encapsulated, tan-pink, soft mass with golden to tan firm, nodular cut-surfaces (Fig. 3A). There was evidence of tumor <1 mm from the resection margin but without evidence of tumor transection. Histopathological examination revealed a variably cellular, well-circumscribed submucosal lesion (Fig. 3B) with bland spindle cells (Fig. 3C), lymphocytes, plasma cells and eosinophils (Fig. 3D). No pleomorphism, mitoses or necroses were identified. Areas with thick-walled vessels were also seen (Fig. 3D). The differential diagnoses included inflammatory fibroid polyp, gastrointestinal stromal tumor (GIST), schwannoma, leiomyoma, perineuroma and solitary fibrous tumor. Immunohistochemistry was negative for CD117, DOG1, Calponin, EMA, SMA, STAT6 and S100. CD34 was found to be positive (Fig. 4). The morphological and immunohistochemical findings supported the diagnosis of inflammatory fibroid polyp. In order to rule out the possibility of GIST, further molecular studies were performed which revealed no mutations in KIT exons 9, 11, 13 and 17 or PDGFRA exons 12, 14 and 18.
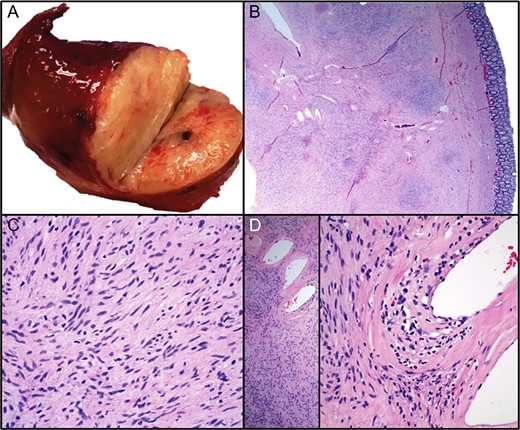
The lesion was grossly a tan-pink, soft mass with golden to tan firm, nodular cut-surfaces ( A). Histopathological examination revealed a variably cellular, well-circumscribed submucosal lesion (B) with bland spindle cells ( C), lymphocytes, plasma cells and eosinophils (D). No pleomorphism, mitoses or necroses were identified. Areas with thick-walled vessels were also seen (D).
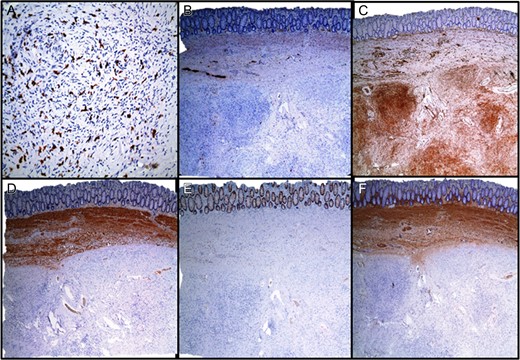
Immunohistochemical staining. (A) CD117, (B) S100, (C) CD34, (D) CALPONIN, (E) EMA, (F) SMA.
The patient subsequently followed up one year later for a screening colonoscopy that demonstrated no evidence of recurrence and no additional masses or polyps.
DISCUSSION
IFPs are rarely found in the rectum, with only few reported cases in the literature [1–5]. The first was described as a 3 cm polypoid mass in an 8-year-old boy who was having bloody stools [4]. The patient ultimately received a resection of the rectum with resultant stenosis. The second case report described an asymptomatic male with a 3 mm submucosal mass demonstrated on colonoscopy that was entirely removed by endoscopic submucosal dissection [5]. The third reported case of IFP describes a 3 cm by 2.7 cm obstructing polypoid mass with mucosal ulceration and presumptive diagnosis of TNM Stage IIIB rectal cancer as there was associated lymph node enlargement and sacral invasion [6]. Our case, however, was a 4.2 cm completely encapsulated lesion that was treated transanally.
Classically, IFPs are histopathologically defined by variable cellularity and vascularity [1–5]. The characteristic perivascular onion-skinning pattern reported in up to 54% cases [3] is extremely helpful though it was absent in our case. Daum et al. previously reported a series of 18 cases lacking this pattern [7]. Variable amounts of inflammatory cells including eosinophils and lymphocytes have been reported in literature. However, a subtype displaying sparse eosinophils along with prominent hyalinization occured in 13% of cases [3]. Our case belongs to this subtype with 3-4 eosinophils per high power field in certain fields and no hyalinization, thus making the diagnosis challenging.
The etiology of IFPs is a controversial topic as they have been linked to inflammatory myofibroblastic tumors [8], mesenchymal tumors such as GISTs [9], PDGFR-A harboring neoplastic conditions as well as familial genetic alterations [10]. Our case displayed immunoreactivity for CD34 showing a linkage to GISTs, though KIT and PDGFR-A mutation screening displayed no mutations.
The presentation of rectal IFPs in the literature is varied and this is the first documented case of an asymptomatic IFP of this size in the rectum. The circumstances are notable as this was a benign lesion that was able to be excised transanally. The benefit of screening colonoscopy cannot be overemphasized, as the patient was not obstructed, anemic, or bleeding per rectum. However, although this is not a malignant tumor, the treatment guidelines have not been standardized. There is no evidence or data to support whether early resection is mandated or watchful waiting in an otherwise asymptomatic patient would be feasible. Biopsy and pathological diagnosis in this case determined the treatment strategy but there is still no standard of care regarding the preferred management paradigm. Considering the presentation of colorectal IFPs of varying sizes, ranging from <1 cm to 7 cm, with some presenting in the context of obstruction or invasion, it follows that early resection would be optimal. However, the recurrence rate for these nonneoplastic lesion is not well studied and further screening recommendations after resection do not currently exist. Further investigation is required to elucidate the appropriate plan of care paradigm for these rare tumors.
CONFLICT OF INTEREST STATEMENT
None declared.
SOURCES OF FUNDING
No funding provided for this report.



