-
PDF
- Split View
-
Views
-
Cite
Cite
Abdulaziz Alshalawi, Ali Alkhaibary, Ali Basalamah, Ali H Alassiri, Abdulaziz Alarifi, Glioblastoma and prolactinoma: a rare simultaneous occurrence, Journal of Surgical Case Reports, Volume 2019, Issue 2, February 2019, rjz030, https://doi.org/10.1093/jscr/rjz030
Close - Share Icon Share
Abstract
The simultaneous development of two or more primary central nervous system (CNS) tumors of different cell types represents 0.9% of all diagnosed CNS tumors. To the best of our knowledge, the simultaneous occurrence of glioblastoma and pituitary adenomas has been reported four times in the English literature, with only two cases harboring prolactinoma and glioblastoma. We report a case of a 42-year-old male who was diagnosed with a sporadic co-occurrence of glioblastoma and a prolactin-secreting pituitary adenoma (prolactinoma). This case report discusses the clinical presentation, radiological/histopathological features, and outcome as well as reviewing the pertinent medical literature. Glioblastoma and a prolactin-secreting adenoma may be detected within the same patient. Further studies are required to delineate the tumorigenesis of the development and co-occurrence of multiple intracranial tumors.
INTRODUCTION
The simultaneous development of two or more primary CNS tumors of different cell origin is extraordinarily rare with an incidence rate of <0.9% of all primary CNS tumors [1]. The most common combination of primary brain tumors is: meningioma and glioma, meningioma and neurinoma, followed by meningioma and pituitary adenoma [2].
Glioblastomas represent around 15–20% of intracranial tumors and account for 50% of all diagnosed gliomas. The co-occurrence of gliomas and pituitary adenomas is extremely rare with only a few cases reported in the literature [2].
Considering the rarity of such co-occurrence, we hereby outline the clinical presentation, radiological/histopathological features and outcome of a 42-year-old male diagnosed with glioblastoma coexisting with a prolactin-secreting pituitary adenoma.
CASE REPORT
A 42-year-old male, medically free, was brought to the emergency department with a month history of poor concentration, blurry vision, poor appetite, left-sided on-and-off numbness, and generalized fatigability. Six months prior to presentation, the patient reported a memory disturbance and sexual dysfunction. There was no history of smoking, radiation exposure or familial-related diseases.
Upon physical examination, the patient was conscious and oriented to time, place and person with a Glasgow Coma Scale (GCS) of 15/15. Examination of the pupils revealed a right pupil (4–5 mm), and a left pupil (3 mm). Visual field examination revealed a right inferior homonymous quadranopsia. The patient was otherwise grossly intact.
Initial laboratory investigations included; a serum prolactin level of 25 526.1 mIU/L (normal range 73–407 mIU/L), an alpha fetoprotein level (AFP) of 3.7 ng/mL (normal range <8 ng/mL), and a total human chorionic gonadotropin (hCG) of <1.2 IU/L (Normal range <5 IU/L).
Radiographic studies showed a centrally necrotic peripherally enhancing mass lesion in the left posteromedial temporal lobe, measuring 3.3 × 3.6 cm2. The non-enhancing component of the tumor extended to the left thalamus. There was a slight signal abnormality in the right thalamus. The tumor itself had a central hemorrhagic component as well. There was an apparent obstruction at the level of the cerebral aqueduct and the third ventricle resulting in a moderate acute hydrocephalus involving the lateral ventricles. The fourth ventricle remained decompressed (Fig. 1a–d).
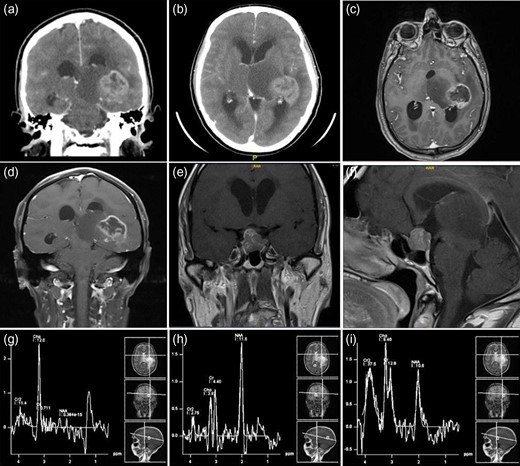
Preoperative neuroimaging of the patient—CT (a and b) and MRI ((c–f )—with contrast enhancement), showing a centrally necrotic peripherally enhancing mass lesion in the left posteromedial temporal lobe representing glioblastoma. The non-enhancing component of the tumor extended to the left thalamus. Another sellar and suprasellar mass lesion representing a pituitary macroadenoma. (g–i) The MR spectroscopy shows a very low N-acetylaspartate (NAA) and high choline keeping up with a malignant tumor.
There was another sellar and suprasellar mass lesion with extension into the right cavernous sinus measuring 1.7 × 1.6 cm2 in the anteroposterior and craniocaudal dimensions, respectively, representing a pituitary macroadenoma. No significant midline shift was noted. The posterior fossa structures were grossly unremarkable. The final impression was an aggressive looking mass lesion involving the left posteromedial temporal lobe and adjacent left thalamus (Fig. 1e and f).
The MR spectroscopy showed a very low N-acetylaspartate (NAA) and high choline keeping up with a malignant tumor (Fig. 1g–i). A contrast-enhanced chest–abdomen, and pelvis CT scan showed no metastasis, lymphadenopathy or any other lesions.
The patient underwent a stereotactic needle biopsy through a temporal burr hole targeting the left posteromedial temporal lobe lesion. Two days post-operation, the patient developed signs and symptoms of hydrocephalus. A repeated brain CT revealed obstructive hydrocephalus and a ventriculoperitoneal shunt was inserted with no postoperative complications.
Hematoxylin and eosin-stained sections prepared from needle biopsy showed a cellular glial neoplasm with high-grade features including significant nuclear atypia, readily identified mitotic activity (Fig. 2a), and microvascular proliferation (Fig. 2b). The glial lineage of this neoplasm was further supported by the positive immunohistochemical reaction to the glial marker Glial Fibrillary Acidic Protein (GFAP; Fig. 2c). As expected, the proliferation index was high and matched the identified high-grade features in this glioblastoma (Fig. 2d). The isocitrate dehydrogenase gene (IDH 1&2) mutation analysis using RT-qPCR technique confirmed the absence of mutation thereby labeling this glioblastoma as an IDH-wildtype. Additionally, methylation analysis for the promoter of methyl guanine methyl transferase (MGMT) revealed the absence of methylation which is considered an unfavorable prognostic factor.
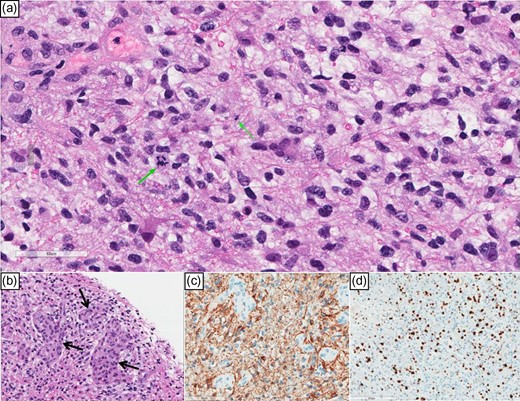
(a) High power view showing hyperchromatic glial nuclei and a couple of mitoses (green arrows). (b) Microvascular proliferation (arrows). (c) (GFAP) immunostain cytoplasmic positivity confirms astrocytic lineage; notice the negatively staining microvascular proliferation. (d) Ki-67 labeling index is high.
The patient was discharged with the same clinical status. He was followed up with medical oncology, radiation oncology and endocrinology.
DISCUSSION
Pituitary adenomas are generally benign slow-growing neoplasms arising from the oral ectoderm [3]. One to two-thirds of which are prolactin-secreting adenomas [3]. Glioblastoma is the most common primary malignant CNS tumor in adults accounting for more than 60% of all primary CNS neoplasms. The global incidence of glioblastoma is 10 per 100 000 people [4]. To the best of our knowledge, the simultaneous occurrence of glioblastoma with pituitary adenomas has been reported four times in the literature, with only two cases harboring prolactinoma and glioblastoma (Table 1). This makes our case the third of its kind.
Summary of the reported cases of the co-occurrence of glioblastoma and pituitary adenomas in the literature
| No. . | Study . | Age/♀/♂ . | Clinical Features . | Labs . | Imaging . | Pathology . | Management . | Follow up . |
|---|---|---|---|---|---|---|---|---|
| 1 | Miyagi et al. [5] | 77/F | Lt. Hemiparesis & vomiting. | –* | Masses in the Rt. frontal lobe, sella turcica, Lt. sphenoidal wing, Lt. anterior clinoidal process and Lt. cerebellar convexity. | GBM, prolactinoma, and transitional meningioma. | – | Death due to pneumonia and cardiac insufficiency 1 month post-operatively. |
| 2 | Furtado et al. [6] | 36/F | Irregular menstrual cycles and generalized tonic-clonic seizures. | – | Rt. frontal parenchymal cyst with a peripheral enhancement. | GBM and prolactinoma. | Radiotherapy followed by TMZ. Bromocriptine. | Recurrence after 8 months at the corpus callosum. |
| 3 | Naydenov et al. [2] | 58/M | Lt. sided Involuntary movements of the face and progressive visual blurring. | – | Heterogeneous Rt. temporal lobe tumor and a suprasellar lesion. | GBM and non-secreting pituitary adenoma. | Conventional external beam RTx plus concomitant and adjuvant TMZ. | Symptom-free for 17 months. Died 3 months later from recurrent GBM. |
| 4 | Haciyakupoglu et al. [1] | 61/M | Headache refractory to Analgesics, nausea, vomiting, and fatigability. | All pituitary hormones within normal ranges. | Rt. frontal tumor mass of and suprasellar mass | GBM and null cell pituitary adenoma. | Dexamethasone, removal of the frontal lesion and subsequent transcranial removal of the suprasellar tumor. | Adjuvant RTx/CMT. Discharged on Day 8. |
| 5 | Present case, 2018 | 42/M | Poor concentration, blurry vision, poor appetite, numbness, sexual dysfunction and generalized fatigability | Prolactinemia | Lt. posteromedial temporal lobe mass and sellar/suprasellar mass lesions. | GBM and prolactinoma. | VP shunt insertion, cabergoline and RTx/CMT. | Discharged and followed up in adult oncology, RTx and endocrinology clinics. |
| No. . | Study . | Age/♀/♂ . | Clinical Features . | Labs . | Imaging . | Pathology . | Management . | Follow up . |
|---|---|---|---|---|---|---|---|---|
| 1 | Miyagi et al. [5] | 77/F | Lt. Hemiparesis & vomiting. | –* | Masses in the Rt. frontal lobe, sella turcica, Lt. sphenoidal wing, Lt. anterior clinoidal process and Lt. cerebellar convexity. | GBM, prolactinoma, and transitional meningioma. | – | Death due to pneumonia and cardiac insufficiency 1 month post-operatively. |
| 2 | Furtado et al. [6] | 36/F | Irregular menstrual cycles and generalized tonic-clonic seizures. | – | Rt. frontal parenchymal cyst with a peripheral enhancement. | GBM and prolactinoma. | Radiotherapy followed by TMZ. Bromocriptine. | Recurrence after 8 months at the corpus callosum. |
| 3 | Naydenov et al. [2] | 58/M | Lt. sided Involuntary movements of the face and progressive visual blurring. | – | Heterogeneous Rt. temporal lobe tumor and a suprasellar lesion. | GBM and non-secreting pituitary adenoma. | Conventional external beam RTx plus concomitant and adjuvant TMZ. | Symptom-free for 17 months. Died 3 months later from recurrent GBM. |
| 4 | Haciyakupoglu et al. [1] | 61/M | Headache refractory to Analgesics, nausea, vomiting, and fatigability. | All pituitary hormones within normal ranges. | Rt. frontal tumor mass of and suprasellar mass | GBM and null cell pituitary adenoma. | Dexamethasone, removal of the frontal lesion and subsequent transcranial removal of the suprasellar tumor. | Adjuvant RTx/CMT. Discharged on Day 8. |
| 5 | Present case, 2018 | 42/M | Poor concentration, blurry vision, poor appetite, numbness, sexual dysfunction and generalized fatigability | Prolactinemia | Lt. posteromedial temporal lobe mass and sellar/suprasellar mass lesions. | GBM and prolactinoma. | VP shunt insertion, cabergoline and RTx/CMT. | Discharged and followed up in adult oncology, RTx and endocrinology clinics. |
*Dashed lines indicate “missing data”.
Abbreviations: M, male; F, female; Rt, right; Lt, left; RTx, radiotherapy; CMT, chemotherapy; VP, ventriculoperitoneal; GBM, glioblastoma; TMZ, temozolomide.
Summary of the reported cases of the co-occurrence of glioblastoma and pituitary adenomas in the literature
| No. . | Study . | Age/♀/♂ . | Clinical Features . | Labs . | Imaging . | Pathology . | Management . | Follow up . |
|---|---|---|---|---|---|---|---|---|
| 1 | Miyagi et al. [5] | 77/F | Lt. Hemiparesis & vomiting. | –* | Masses in the Rt. frontal lobe, sella turcica, Lt. sphenoidal wing, Lt. anterior clinoidal process and Lt. cerebellar convexity. | GBM, prolactinoma, and transitional meningioma. | – | Death due to pneumonia and cardiac insufficiency 1 month post-operatively. |
| 2 | Furtado et al. [6] | 36/F | Irregular menstrual cycles and generalized tonic-clonic seizures. | – | Rt. frontal parenchymal cyst with a peripheral enhancement. | GBM and prolactinoma. | Radiotherapy followed by TMZ. Bromocriptine. | Recurrence after 8 months at the corpus callosum. |
| 3 | Naydenov et al. [2] | 58/M | Lt. sided Involuntary movements of the face and progressive visual blurring. | – | Heterogeneous Rt. temporal lobe tumor and a suprasellar lesion. | GBM and non-secreting pituitary adenoma. | Conventional external beam RTx plus concomitant and adjuvant TMZ. | Symptom-free for 17 months. Died 3 months later from recurrent GBM. |
| 4 | Haciyakupoglu et al. [1] | 61/M | Headache refractory to Analgesics, nausea, vomiting, and fatigability. | All pituitary hormones within normal ranges. | Rt. frontal tumor mass of and suprasellar mass | GBM and null cell pituitary adenoma. | Dexamethasone, removal of the frontal lesion and subsequent transcranial removal of the suprasellar tumor. | Adjuvant RTx/CMT. Discharged on Day 8. |
| 5 | Present case, 2018 | 42/M | Poor concentration, blurry vision, poor appetite, numbness, sexual dysfunction and generalized fatigability | Prolactinemia | Lt. posteromedial temporal lobe mass and sellar/suprasellar mass lesions. | GBM and prolactinoma. | VP shunt insertion, cabergoline and RTx/CMT. | Discharged and followed up in adult oncology, RTx and endocrinology clinics. |
| No. . | Study . | Age/♀/♂ . | Clinical Features . | Labs . | Imaging . | Pathology . | Management . | Follow up . |
|---|---|---|---|---|---|---|---|---|
| 1 | Miyagi et al. [5] | 77/F | Lt. Hemiparesis & vomiting. | –* | Masses in the Rt. frontal lobe, sella turcica, Lt. sphenoidal wing, Lt. anterior clinoidal process and Lt. cerebellar convexity. | GBM, prolactinoma, and transitional meningioma. | – | Death due to pneumonia and cardiac insufficiency 1 month post-operatively. |
| 2 | Furtado et al. [6] | 36/F | Irregular menstrual cycles and generalized tonic-clonic seizures. | – | Rt. frontal parenchymal cyst with a peripheral enhancement. | GBM and prolactinoma. | Radiotherapy followed by TMZ. Bromocriptine. | Recurrence after 8 months at the corpus callosum. |
| 3 | Naydenov et al. [2] | 58/M | Lt. sided Involuntary movements of the face and progressive visual blurring. | – | Heterogeneous Rt. temporal lobe tumor and a suprasellar lesion. | GBM and non-secreting pituitary adenoma. | Conventional external beam RTx plus concomitant and adjuvant TMZ. | Symptom-free for 17 months. Died 3 months later from recurrent GBM. |
| 4 | Haciyakupoglu et al. [1] | 61/M | Headache refractory to Analgesics, nausea, vomiting, and fatigability. | All pituitary hormones within normal ranges. | Rt. frontal tumor mass of and suprasellar mass | GBM and null cell pituitary adenoma. | Dexamethasone, removal of the frontal lesion and subsequent transcranial removal of the suprasellar tumor. | Adjuvant RTx/CMT. Discharged on Day 8. |
| 5 | Present case, 2018 | 42/M | Poor concentration, blurry vision, poor appetite, numbness, sexual dysfunction and generalized fatigability | Prolactinemia | Lt. posteromedial temporal lobe mass and sellar/suprasellar mass lesions. | GBM and prolactinoma. | VP shunt insertion, cabergoline and RTx/CMT. | Discharged and followed up in adult oncology, RTx and endocrinology clinics. |
*Dashed lines indicate “missing data”.
Abbreviations: M, male; F, female; Rt, right; Lt, left; RTx, radiotherapy; CMT, chemotherapy; VP, ventriculoperitoneal; GBM, glioblastoma; TMZ, temozolomide.
Miyagi et al. [5] described a case of a 77-year-old female diagnosed with multicentric glioblastoma and prolactinoma. Furtado et al. [6] reported a case of glioblastoma coexisting with prolactinoma. Since then, only three more cases of gliomas co-occurring with pituitary adenomas have been reported. Naydenov et al. [2] reported a case of non-familial Turcot’s syndrome that was associated with colon cancer, glioblastoma and pituitary adenoma.
In the present case, the patient simultaneously developed two distinct tumors that are separate from each other (no collision). This co-occurrence was neither familial nor syndromic with no risk factors identified. Although no tissue biopsy was performed on the sellar-suprasellar tumor to confirm that it was a prolactinoma, the clinical picture, laboratory investigations, and radiological findings were suggestive of a prolactin-secreting adenoma.
To date, no causative factors have been identified to explain the co-occurrence of these unrelated brain tumors. However, the low incidence of this phenomenon might raise the possibility of a common genetic linkage to these non-syndromic tumors [6].
The diagnosis of multiple intracranial neoplasms requires a high index of suspicion. This can be suggested by the inconsistency between clinical and radiological features, the unexpected presence of a second mass during surgical intervention or autopsy, the unexpected postoperative deterioration, the and the presence of signs and symptoms of recurrence especially after operating on one of tumors [7].
Rarely, co-existence of glioblastoma and prolactinoma may occur. In patients diagnosed with glioblastoma, extremely high levels of prolactin in conjunction with a radiological confirmation of suprasellar/sellar lesions might raise the possibilities of the simultaneous occurrence of glioblastoma and prolactinoma.
Conflict of Interest statement
Nil.
Disclosure
Nil.
Financial Support
Nil.



