-
PDF
- Split View
-
Views
-
Cite
Cite
Yasuhiro Tanaka, Shien Seike, Koichi Tomita, Jun-ichiro Ikeda, Eiichi Morii, Emiko Tanaka Isomura, Tateki Kubo, Possible malignant transformation of arteriovenous malformation to angiosarcoma: case report and literature review, Journal of Surgical Case Reports, Volume 2019, Issue 12, December 2019, rjz375, https://doi.org/10.1093/jscr/rjz375
Close - Share Icon Share
Abstract
Angiosarcoma is a rare malignant tumour, which accounts for 1–2% of all malignant soft-tissue tumours. Most cases of angiosarcoma arise spontaneously, and malignant transformation of vascular malformation to angiosarcoma is extremely rare. We describe the case of a 70-year-old woman with a massive arteriovenous malformation in her shoulder, which gradually enlarged, despite repeated surgeries and radiation therapy over 53 years. She also presented with rapidly growing haemorrhagic masses in her oral cavity. Excision biopsy was performed, and the pathohistological diagnosis was angiosarcoma. Positron emission tomography–computed tomography revealed high fluorodeoxyglucose accumulation in the oral cavity and right shoulder, the latter of which was consistent with the location of the arteriovenous malformation. The masses in the oral cavity were diagnosed as metastatic angiosarcoma from the right shoulder, where the massive arteriovenous malformation was suspected to have malignantly transformed. This report describes a possible case of malignant transformation of arteriovenous malformation to angiosarcoma.
Introduction
Angiosarcoma (AS) is a rare soft-tissue sarcoma of endothelial cell origin with a poor prognosis, accounting for 1–2% of all soft-tissue sarcomas. AS is rarely diagnosed in the oral cavity (1), and most cases of AS arise spontaneously. As with other soft-tissue sarcomas, the role of precursor malignancies remains unclear. Although several cases of AS arising from vascular anomalies have been reported, precursor lesions are often unclear because primary lesions were not described using the International Society for the Study of Vascular Anomalies (ISSVA) classification. The ISSVA classification categorizes vascular anomalies as vascular tumours that present as neoplastic growths and vascular malformations that have abnormal anastomoses and structures in vessels with poor proliferative ability. Arteriovenous malformation (AVM) is classified as a vascular malformation, and malignant transformation of AVM to AS is extremely rare, with only one case report to date demonstrating AS arising from AVM (2). The present case is the second to describe the possibility of malignant transformation of AVM to AS.
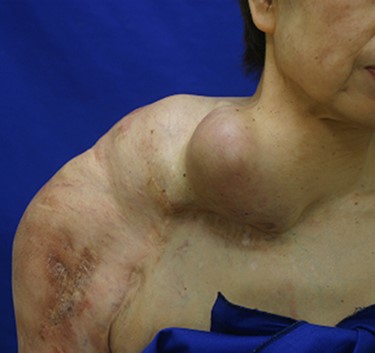
Arteriovenous malformation (AVM) in the right upper limb reaches to the shoulder.
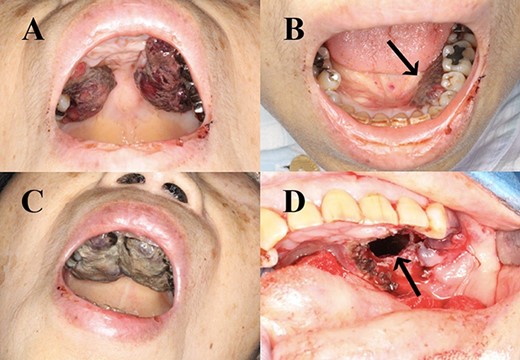
Haemorrhagic masses in the oral cavity. These were identified on the hard palate (A) and the left lower gingiva (arrow) (B). Two weeks after initial presentation (C). Spreading to the upper gingiva, maxillary bone and maxillary sinus (arrow; after resection) (D).
Case report
A 70-year-old Japanese woman was referred to our department. Her medical history indicated surgery, radiation therapy and intravascular treatment for AVM in her right upper limb since she was 17 years old (Fig. 1). One month before initial presentation, she noticed frequent haemorrhagic masses in her oral cavity. She complained of general malaise and loss of appetite. The masses bled readily upon scraping the hard palate (Fig. 2A). Similar masses were found on her left lower gingiva (Fig. 2B). Routine blood tests revealed normocytic normochromic anaemia of 4.6 g/dl, while no platelet or coagulation abnormalities were found. Open incisional biopsy under general anaesthesia was performed after conservative management, including blood transfusion, nutrition and oral care. The masses had grown rapidly and covered the hard palate and had spread to the upper gingiva, maxillary bone and maxillary sinus (Fig. 2C and D). The specimen consisted of bloody, dark-red and partially sponge-like lesions. Histologically, necrosis and haemorrhage were evident. In areas where viable cells were present, atypical, vascular endothelial cells with pleomorphic nuclei formed a solid lesion or a network to form a narrow lumen filled with blood cells (Fig. 3). Immunohistochemical staining was positive for CD31, CK (AE1 + 3), ERG, D2–40 and CK (CAM 5.2) and negative for CD34, FactorVIII, αSMA, MyoD1, Desmin, Myogenin, S100, HMB45, MelanA, CK (5/6), p63, p40 and EMA. MIB-1 staining showed a proliferation index as high as 50%. These pathohistological findings revealed the tumour as AS (FNCLCC Histological Grade 3).
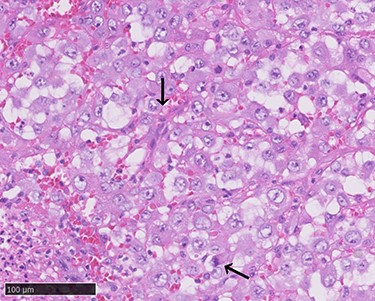
Atypical proliferation of dedifferentiated cells with pleomorphic nuclei and atypical mitotic figures (arrows, haematoxylin–eosin stain, ×400).
Positron emission tomography–computed tomography (CT) revealed high fluorodeoxyglucose (FDG) accumulation in her oral cavity and right shoulder in accordance with the location of the AVM (Fig. 4). High accumulation was also evident in her left lung, lumbar spine, piriformis and lymph nodes, suggesting multiple metastases. We diagnosed the masses in the oral cavity as metastatic AS, with the primary tumour located in the right shoulder, where the massive AVM was suspected to have malignantly transformed. The patient desired the best supportive care and declined additional treatments such as radiotherapy and chemotherapy. She died 4 months after initially noticing the intraoral masses. An autopsy was not performed.
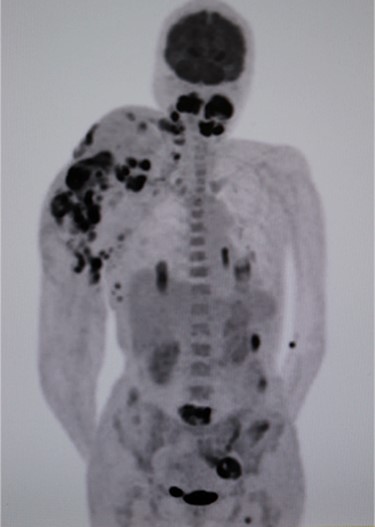
Positron emission tomography–computed tomography (PET-CT). High fluorodeoxyglucose (FDG) accumulation in the oral cavity as well as in the right shoulder where the AVM was located. High accumulation was also evident in the left lung, lumbar spine, piriformis and lymph nodes.
Discussion
AS is an aggressive malignant tumour with high metastatic potential. Main metastatic sites of AS include the lungs (25%), bone (22%), liver (16%) and brain (11%) (3). Intraoral metastatic AS is rare but tends to localize in the gingiva (4). As is often the case with oral metastases from other primary lesions, their presence is evidence of widespread disease. Although we could not identify the primary lesion in the right shoulder by visual inspection or CT scan due to the massive AVM, we considered it highly unlikely that the masses in the oral cavity formed the primary lesion. Only a few studies have reported on primary intraoral AS with multiple lesions, and the widest range of high FDG accumulation in our case was observed in the right shoulder.
Although most ASs arise spontaneously, AS can arise from pre-existing benign vascular lesions, particularly in cases with a history of radiation therapy, which is a well-known risk factor of AS. One report described a case of AS arising from AVM, in which AS arose from a frontoparietal AVM which had been identified many years prior (2). This patient had no history of radiation therapy but had received prednisolone and budesonide for collagenous colitis. In another study, AS arising from AV fistulas was described in a kidney transplant patient on immunosuppression who had received an artificial arteriovenous shunt (5). Although development of AS from AVM seems to be less likely without previous radiation or immunosuppression, more than 10 studies have reported cases of AS arising in the setting of benign vascular lesions without any risk factors. One case showed AS arising within a cavernous haemangioma with a 10-year latency period between the development of the precursor lesion and its transformation to AS (6).
Hua et al. (7) reported AS arising from lymphatic-venous malformation (LVM) without any risk factors. At first glance, this report may seem consistent with past reports of AS arising from benign vascular lesions without any risk factors. However, it differs in that the pre-existing lesion was diagnosed as LVM. In most previous reports, the word ‘haemangioma’ is used to indicate benign vascular lesions and never to describe any specific type of vascular tumours or malformations, in accordance with the ISSVA classification. The ISSVA categorizes vascular anomalies into vascular tumours and malformations. According to the ISSVA classification, haemangioma refers to endothelial cell-proliferative, neoplastic lesions, while vascular malformation is characterized by progressing with growth over congenital angiogenesis without cell proliferation. To elucidate the possibility of AS arising from vascular anomalies, the existing lesions must be declared precisely according to the ISSVA classification.
In our case, the location of the primary lesion could not be clarified given the existence of multiple metastases at the time of diagnosis. However, it is reasonable to speculate that AS arose from the shoulder AVM, where the widest range of FDG accumulation was observed. Careful monitoring of AS occurrence is needed in patients with vascular malformation, particularly those with risk factors such as a history of radiation therapy and immunosuppression.
Conflict of interest statement
None declared.



