-
PDF
- Split View
-
Views
-
Cite
Cite
Antoon H van Lierop, Peter H Bisschop, Anita Boelen, Susanne van Eeden, Anton F Engelman, Elisabeth J Nieveen van Dijkum, Heinz-Josef Klümpen, hypercalcaemia due to a calcitriol-producing neuroendocrine tumour, Journal of Surgical Case Reports, Volume 2019, Issue 12, December 2019, rjz346, https://doi.org/10.1093/jscr/rjz346
Close - Share Icon Share
Abstract
In this case report, we describe a 40-year-old patient with a large grade 2 pancreatic neuroendocrine tumour (pNET) with spleen metastasis. Albeit radical resection, he developed liver metastasis after 2 years, for which he underwent radio frequency ablation and embolization, and was treated successfully with different subsequent lines of systemic therapy. Eight years after the initial diagnosis, he was admitted for symptomatic and refractory hypercalcaemia, due to calcitriol synthesis by the liver metastasis. After tumour load reduction by hemihepatectomy, there was an initial normalization of hypercalcaemia, until it recurred after 18 months. In this period, the liver metastasis had progressed despite chemo- and immunotherapy. Patient underwent an additional extend hemihepatectomy, from which he recovered well with normalization of calcium levels. This case illustrates the hormonal plasticity of pNETs and shows how prolonged survival can be achieved for metastatic pNET by multimodality approach.
INTRODUCTION
Pancreatic neuroendocrine tumours (pNETs) are rare neoplasms that arise from islet cells. Hormonal syndromes are present in ~30% of pNETs [1]. Although these are generally present at diagnosis, secondary hormonal secretion develops in minority of patients [2, 3]. Here we describe a patient with a nonfunctional hepatogenic metastasized pNET, who developed excessive calcitriol synthesis 10 years after diagnosis, leading to refractory symptomatic hypercalcaemia.
CASE REPORT
The patient is a 50-year-old Caucasian male. His medical history was insignificant up to the age of 40 years, when he was diagnosed with a nonfunctional pNET of 11.5 cm with splenic metastasis of 1.4 cm, for which he underwent combined pancreatic tail resection and splenectomy (Fig. 1). Histological examination showed well-differentiated grade 2 neuroendocrine tumours (Ki-67 index 10%), radically resected (R0), and one positive lymph node (T4N1M1, stage IV).
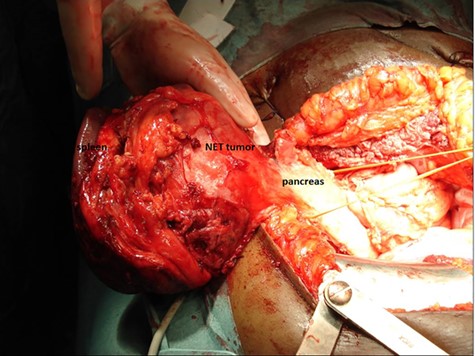
pNET with spleen metastasis, resected by combined pancreatic tail resection and splenectomy.
After 2 years, patient developed liver metastasis in segments 5/6 (Ki index 10–20%, grade 2). He received radio frequency ablation and embolization and was treated with somatuline 120 mg monthly. Because of disease progression, he was subsequently treated successfully in a phase II clinical trial with BEZ235 [4] and later on palliative treatment with streptozocin/5-fluorouracil.
A year after his last chemotherapy, 8 years after diagnosis, he was admitted for severe symptomatic hypercalcaemia. Laboratory results are summarized in Table 1 below. In brief, albumin-corrected serum calcium was 3.49 mmol/l, while levels of parathyroid hormone (PTH) were low (0.76 pmol/l) and parathyroid hormone-related peptide (PTHrP) was undetectable (<0.3 pmol/l). In contrast, levels of 1,25 dihydroxycholecalciferol (1,25(-OH)2D3) were increased (342 pmol/l). Levels of Thyroid Stimulating Hormone (TSH) and Angiotensin-converting Enzyme (ACE) were normal, and there was no M-protein. The hypercalcaemia was resistant to zoledronate but declined after hyperhydration and dietary calcium restriction.
| Date . | Admission . | Day 7 . | Dag 14 . | Post-operative . | Reference range . |
|---|---|---|---|---|---|
| Calcium (mmol/l) | 349 | 287 | 348 | 243 | 210–255 |
| Phosphate (mmol/l) | 0.78 | 0.90 | 0.76 | 1.16 | 0.90–1.50 |
| Creatinine (umol/l) | 70 | 64 | 70 | 75 | 80–125 |
| PTH (pmol/l) | 0.76 | 417 | 0.6–6.7 | ||
| PTHrP (pmol/l) | <0.3 | <0.6 | |||
| 25(OH)D3 (nmol/l) | 64 | 75–250 | |||
| 1,25(OH)2D3 (pmol/l) | 342 | 433 | 111 | 59–159 |
| Date . | Admission . | Day 7 . | Dag 14 . | Post-operative . | Reference range . |
|---|---|---|---|---|---|
| Calcium (mmol/l) | 349 | 287 | 348 | 243 | 210–255 |
| Phosphate (mmol/l) | 0.78 | 0.90 | 0.76 | 1.16 | 0.90–1.50 |
| Creatinine (umol/l) | 70 | 64 | 70 | 75 | 80–125 |
| PTH (pmol/l) | 0.76 | 417 | 0.6–6.7 | ||
| PTHrP (pmol/l) | <0.3 | <0.6 | |||
| 25(OH)D3 (nmol/l) | 64 | 75–250 | |||
| 1,25(OH)2D3 (pmol/l) | 342 | 433 | 111 | 59–159 |
| Date . | Admission . | Day 7 . | Dag 14 . | Post-operative . | Reference range . |
|---|---|---|---|---|---|
| Calcium (mmol/l) | 349 | 287 | 348 | 243 | 210–255 |
| Phosphate (mmol/l) | 0.78 | 0.90 | 0.76 | 1.16 | 0.90–1.50 |
| Creatinine (umol/l) | 70 | 64 | 70 | 75 | 80–125 |
| PTH (pmol/l) | 0.76 | 417 | 0.6–6.7 | ||
| PTHrP (pmol/l) | <0.3 | <0.6 | |||
| 25(OH)D3 (nmol/l) | 64 | 75–250 | |||
| 1,25(OH)2D3 (pmol/l) | 342 | 433 | 111 | 59–159 |
| Date . | Admission . | Day 7 . | Dag 14 . | Post-operative . | Reference range . |
|---|---|---|---|---|---|
| Calcium (mmol/l) | 349 | 287 | 348 | 243 | 210–255 |
| Phosphate (mmol/l) | 0.78 | 0.90 | 0.76 | 1.16 | 0.90–1.50 |
| Creatinine (umol/l) | 70 | 64 | 70 | 75 | 80–125 |
| PTH (pmol/l) | 0.76 | 417 | 0.6–6.7 | ||
| PTHrP (pmol/l) | <0.3 | <0.6 | |||
| 25(OH)D3 (nmol/l) | 64 | 75–250 | |||
| 1,25(OH)2D3 (pmol/l) | 342 | 433 | 111 | 59–159 |
On 68Ga-DOTATATE-PET and 18F-FDG-PET scans increased volume of liver metastasis in segment 5/6, and an enlarged portocaval lymph node was seen with increased metabolic activity only on 18F-FDG-PET scan. Histological biopsies of the liver and lymph node showed grade 2 pNET (Ki-67 15–20%) and normal lymphoid tissue, respectively.
Due to aberrant arterial supply of the liver tumour, embolization was not possible.
Instead patient underwent a hemihepatectomy of segments 4b/5/6 and portocaval lymph node dissection to achieve tumour load reduction. Pathological examination showed a radically resected, well-differentiated NET grade 2 (Ki-67 index <20%), with areas of higher proliferation (grade 3, Ki-67 index 20–25%).
The patient’s condition improved rapidly after the operation, including normalisation of calcium and 1,25(OH)2D3 levels.
To better understand the cause of the hypercalcaemia, we determined in tumour tissue mRNA expression of CYP27B1, which encodes 25-hydroxyvitamin D3 1-alpha-hydroxylase, the enzyme that converts 25-hydroxyvitamin D3 into the active metabolite 1,25(OH)2D3. The relative expression of CYP27B1 in tumour tissue was 1000-fold higher compared to control samples of human foetal kidney cells and lymphoblast cells, known to express 1-alpha-hydroxylase.
Unfortunately, 3 months after hemihepatectomy, CT imaging revealed new peritoneal and hepatic metastasis. After 4 months of treatment with capecitabine and temozolomide, he was switched to immunotherapy with nivolumab within the Drug Rediscovery Protocol (DRUP trial; ClinicalTrials.gov Identifier: NCT02925234), after DNA profiling of tumour biopsy had shown high mutation load.
While peritoneal metastases declined under therapy, the hepatic mass increased. Eight months after initiation of nivolumab (18 months after the first episode of hypercalcaemia), patient was readmitted for hypercalcaemia.
On CT imaging the liver mass in segment 5/8 had increased to 15.3 cm. Hepatobiliary scan showed sufficient liver function of segments 1, 2, 3 and 4a. After vena porta embolization, he underwent a palliative extended hemihepatectomy. Pathological examination showed a R1 resected 12 cm liver NET grade 3, with a Ki-67 index of 40%, and necrotic mesenteric metastasis without vital tumour cells.
Patient recovered well from the operation. His liver function remained normal and his calcium levels normalized. At time of manuscript submission, patient was still alive 10 years after diagnosis.
DISCUSSION
Here we described a patient with metastasized pNET, dedifferentiating after 10 years, with increased proliferation and ectopic synthesis of calcitriol, causing refractory hypercalcaemia. Secondary hormonal synthesis develops in 4% of NETs [2, 3]. The pathogenesis of this metachronous hormonal synthesis is poorly understood but might be due to selection of different tumour cell clones, induced by antitumour treatments [2, 3]. Retrospective studies have shown hormone synthesis is associated with disease progression, higher Ki-67 index, and mortality [2, 3].
hypercalcaemia is a frequently encountered complication in malignancy. It is predominantly caused by the secretion of PTHrP by solid tumours or leukemic cells or by the presence of osteolytic lesions. While hypercalcaemia due to increased calcitriol levels can be found in lymphomas, it is more often caused by granulomatous diseases as sarcoidosis or tuberculosis.
In our patient, there were no signs of sarcoidosis, tuberculosis, or lymphoma on imaging and laboratory investigations. In contrast, we found elevated expression of CYP27B1 in tumour tissue, suggesting that the high calcitriol levels were generated by the pNET, which is further supported by the normalization of calcitriol levels after hemihepatectomy.
In contrast to other tumour types, hypercalcaemia is uncommon in NETs [5]. Less than 30 cases of PTHrP-producing NETs have been reported [6] and 1 with a PTH-secreting pNET [7]. We have found only one other case in literature of a calcitriol-secreting NET [8]. This case also concerned a metastasized pNET in which the calcitriol synthesis developed later, 4 years into the course of the disease, and after treatment with octreotide.
This case report highlights the hormonal plasticity of a pNET and stresses the importance of hormonal screening in patients with initially nonfunctional pNETs when they develop new symptoms. It further shows that by multimodality approach, prolonged survival can be achieved for metastatic pNETs.



