-
PDF
- Split View
-
Views
-
Cite
Cite
Catharine Kappauf, Jamal Rahaman, Daniel Popowich, Abdominal cocoon: an unexpected cause of ascites in a healthy patient, Journal of Surgical Case Reports, Volume 2019, Issue 12, December 2019, rjz310, https://doi.org/10.1093/jscr/rjz310
Close - Share Icon Share
Abstract
Abdominal cocoon is the idiopathic fibrotic encasement of abdominal organs. It classically presents as small bowel obstruction in young women. In this case report, we present a rare example of a patient presenting solely with massive ascites of presumed gynecologic origin, who upon surgical exploration was found to have abdominal cocoon. We discuss the patient’s unique disease presentation, unrevealing work-up and the treatment strategy pursued, and provide a review of the literature.
INTRODUCTION
Abdominal cocoon, or idiopathic encapsulating sclerosing peritonitis (IESP), is a rare disease entity first described by Foo et al. in 1978 [1]. The exact incidence is unknown and difficult to ascertain given conflicting definitions in the literature, but it is estimated that fewer than 200 cases have been reported [2]. IESP is characterized by full or partial encapsulation of small bowel and possibly other abdominal organs by a fibrous membrane [1]. While IESP has no known etiology, secondary sclerosing encapsulating peritonitis has been associated with peritoneal dialysis, recurrent peritonitis, luteinized thecomas, abdominal tuberculosis, autoimmune diseases and several other causes [3–5]. Preoperative diagnosis is difficult, owing to disease rarity, nonspecific imaging findings and its varied clinical presentation, which may range from abdominal pain to small bowel obstruction [3, 6]. This case report is a rare example of abdominal cocoon presenting as massive ascites in the absence of bowel symptoms or exposures that would contribute to secondary sclerosing peritonitis [7].
CASE REPORT
A 31-year-old healthcare worker with no medical history presented to her gynecologist complaining of 2 months of abdominal bloating and increasing abdominal girth. She denied nausea, vomiting, abdominal pain, changes in bowel habits, fevers or chills. She was taking iron supplements only. She denied family history of gynecologic or gastrointestinal cancer, recent or distant travel, and had always had negative PPD tests. Review of systems was unrevealing. Physical exam was notable for a tense, nontender, distended abdomen. CBC showed WBC count 2.4 × 103 without other abnormalities (Table 1). Blood chemistries, liver function tests, amylase and lipase were within normal range. CA 19–9 was slightly elevated at 40.3 (N < 35) and other tumor markers including AFP, CA125, CEA, CA 27–29 and albumin were within the normal range. Ultrasound and noncontrast CT of the abdomen and pelvis revealed massive ascites, as well as displacement of abdominal organs superiorly and inferiorly to the ascitic fluid (Fig. 1). All organs appeared normal, but evaluation was limited by the mass effect of the ascites. Aside from an endometrial polyp, there was no evidence of abdominal masses or nodules.
| Lab . | Initial presentation . | 7-month f/u . | Normal range . |
|---|---|---|---|
| WBC | 2.4 | 4.1 | 3.4–10.8 × 103/ul |
| Hgb | 11.1 | 11.5 | 11.1–15.9 g/dl |
| Hct | 35 | 36.6 | 34–47% |
| Plt | 327 | 254 | 150–450 × 103/ul |
| Ca2+ | 9.3 | 8.7–10.2 mg/dl | |
| Amylase | 93 | 31–124 U/l | |
| Lipase | 42 | 14–72 | |
| ALT | 13 | 18 | 0–32 U/l |
| AST | 21 | 25 | 0–40 U/l |
| Total bilirubin | 0.3 | 0.6 | 0–1.2 |
| Direct bilirubin | 0.1 | 0–0.4 | |
| Alk phos | 30 | 28 | 39–117 U/l |
| LDH | 175 | 100–220 U/l | |
| Albumin | 3.9 | 4 | 3.5–4.9 g/dl |
| AFP | 3.7 | 0–9 ng/ml | |
| CA 19–9 | 40.3 | 22.2 | 0–35 U/ml |
| CA 125 | 16 | 14 | 0–35 U/ml |
| CEA | 1 | 1.2 | 0–3 ng/ml |
| CA 27.29 | 33.1 | 0–38.6 U/ml | |
| Inhibin B | 159.8 | Not reported | |
| APTT | 28.5 | 25.4–34.9 s | |
| PT | 13.6 | 12.3–14.9 s | |
| INR | 1 | 1 |
| Lab . | Initial presentation . | 7-month f/u . | Normal range . |
|---|---|---|---|
| WBC | 2.4 | 4.1 | 3.4–10.8 × 103/ul |
| Hgb | 11.1 | 11.5 | 11.1–15.9 g/dl |
| Hct | 35 | 36.6 | 34–47% |
| Plt | 327 | 254 | 150–450 × 103/ul |
| Ca2+ | 9.3 | 8.7–10.2 mg/dl | |
| Amylase | 93 | 31–124 U/l | |
| Lipase | 42 | 14–72 | |
| ALT | 13 | 18 | 0–32 U/l |
| AST | 21 | 25 | 0–40 U/l |
| Total bilirubin | 0.3 | 0.6 | 0–1.2 |
| Direct bilirubin | 0.1 | 0–0.4 | |
| Alk phos | 30 | 28 | 39–117 U/l |
| LDH | 175 | 100–220 U/l | |
| Albumin | 3.9 | 4 | 3.5–4.9 g/dl |
| AFP | 3.7 | 0–9 ng/ml | |
| CA 19–9 | 40.3 | 22.2 | 0–35 U/ml |
| CA 125 | 16 | 14 | 0–35 U/ml |
| CEA | 1 | 1.2 | 0–3 ng/ml |
| CA 27.29 | 33.1 | 0–38.6 U/ml | |
| Inhibin B | 159.8 | Not reported | |
| APTT | 28.5 | 25.4–34.9 s | |
| PT | 13.6 | 12.3–14.9 s | |
| INR | 1 | 1 |
| Lab . | Initial presentation . | 7-month f/u . | Normal range . |
|---|---|---|---|
| WBC | 2.4 | 4.1 | 3.4–10.8 × 103/ul |
| Hgb | 11.1 | 11.5 | 11.1–15.9 g/dl |
| Hct | 35 | 36.6 | 34–47% |
| Plt | 327 | 254 | 150–450 × 103/ul |
| Ca2+ | 9.3 | 8.7–10.2 mg/dl | |
| Amylase | 93 | 31–124 U/l | |
| Lipase | 42 | 14–72 | |
| ALT | 13 | 18 | 0–32 U/l |
| AST | 21 | 25 | 0–40 U/l |
| Total bilirubin | 0.3 | 0.6 | 0–1.2 |
| Direct bilirubin | 0.1 | 0–0.4 | |
| Alk phos | 30 | 28 | 39–117 U/l |
| LDH | 175 | 100–220 U/l | |
| Albumin | 3.9 | 4 | 3.5–4.9 g/dl |
| AFP | 3.7 | 0–9 ng/ml | |
| CA 19–9 | 40.3 | 22.2 | 0–35 U/ml |
| CA 125 | 16 | 14 | 0–35 U/ml |
| CEA | 1 | 1.2 | 0–3 ng/ml |
| CA 27.29 | 33.1 | 0–38.6 U/ml | |
| Inhibin B | 159.8 | Not reported | |
| APTT | 28.5 | 25.4–34.9 s | |
| PT | 13.6 | 12.3–14.9 s | |
| INR | 1 | 1 |
| Lab . | Initial presentation . | 7-month f/u . | Normal range . |
|---|---|---|---|
| WBC | 2.4 | 4.1 | 3.4–10.8 × 103/ul |
| Hgb | 11.1 | 11.5 | 11.1–15.9 g/dl |
| Hct | 35 | 36.6 | 34–47% |
| Plt | 327 | 254 | 150–450 × 103/ul |
| Ca2+ | 9.3 | 8.7–10.2 mg/dl | |
| Amylase | 93 | 31–124 U/l | |
| Lipase | 42 | 14–72 | |
| ALT | 13 | 18 | 0–32 U/l |
| AST | 21 | 25 | 0–40 U/l |
| Total bilirubin | 0.3 | 0.6 | 0–1.2 |
| Direct bilirubin | 0.1 | 0–0.4 | |
| Alk phos | 30 | 28 | 39–117 U/l |
| LDH | 175 | 100–220 U/l | |
| Albumin | 3.9 | 4 | 3.5–4.9 g/dl |
| AFP | 3.7 | 0–9 ng/ml | |
| CA 19–9 | 40.3 | 22.2 | 0–35 U/ml |
| CA 125 | 16 | 14 | 0–35 U/ml |
| CEA | 1 | 1.2 | 0–3 ng/ml |
| CA 27.29 | 33.1 | 0–38.6 U/ml | |
| Inhibin B | 159.8 | Not reported | |
| APTT | 28.5 | 25.4–34.9 s | |
| PT | 13.6 | 12.3–14.9 s | |
| INR | 1 | 1 |
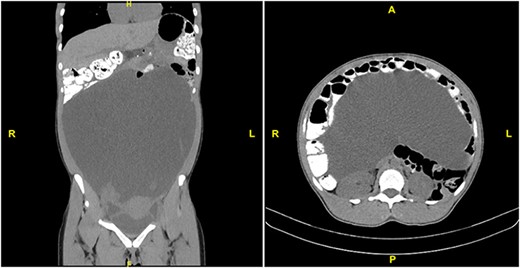
CT scan with PO and IV contrast demonstrating massive ascites and displacement of abdominal viscera. Left: coronal section. Right: transverse section at L1.
Attempts at in-office paracentesis were unsuccessful, so the decision was made to pursue diagnostic laparoscopy for drainage of ascites, dilation and curettage to remove the endometrial polyp, and possible appendectomy given concern for pseudomyxoma peritonei. Intraoperatively, 7 l of green ascitic fluid was drained from the abdominal cavity through an infraumbilical incision. Subsequent laparoscopic exploration of the peritoneal cavity revealed abdominal cocoon syndrome, evidenced by fibrotic encasement and displacement of all abdominal organs. The uterus, fallopian tubes and ovaries were covered by dense adhesions and inflammatory exudate. There was no visible rectum or sigmoid colon, and the entire large bowel appeared to be displaced retroperitoneally, covered with fibrotic peritonitis. No aspect of the small or large bowel could be visualized, nor could the liver, stomach or spleen. There was a thick film of fibrous tissue immediately cephalad to the umbilicus, which appeared to sequester the small bowel in the upper abdomen. The colorectal surgery team was consulted intraoperatively to confirm the diagnosis and assist in evaluation of the abdominal viscera. Biopsies of the peritoneum were obtained with great care, and no attempt was made to resect the extensive fibrotic adhesions. Finally, the endometrial polyp was removed via dilation and curettage.
The ascitic fluid was sent for cytology, gram stain, fungal and TB cultures. Cultures were negative, and cytology showed no evidence of malignancy (Table 2). Pathologic evaluation of the endometrial curetting showed a benign polyp and proliferative endometrium. Peritoneal biopsy specimens demonstrated benign fibrous material, chronic inflammation and reactive mesothelial cells, confirming the diagnosis of abdominal cocoon syndrome.
Results from pathologic and cytologic analysis following each surgical procedure
| Test . | Result: index procedure . | Result: repeat procedure . |
|---|---|---|
| Ascites cytology | Benign | Benign |
| Gram stain | Negative | Negative |
| Cultures (fungal, TB) | Negative | Negative |
| Peritoneal biopsy | ‐ Benign fibrous material | Findings similar to index procedure |
| ‐ chronic inflammation | ||
| ‐ reactive mesothelial cells | ||
| Immunostaining | ||
| ‐ AE1/3 | Not performed | + |
| ‐ Calretinin | + | |
| ‐ CD10 | + |
| Test . | Result: index procedure . | Result: repeat procedure . |
|---|---|---|
| Ascites cytology | Benign | Benign |
| Gram stain | Negative | Negative |
| Cultures (fungal, TB) | Negative | Negative |
| Peritoneal biopsy | ‐ Benign fibrous material | Findings similar to index procedure |
| ‐ chronic inflammation | ||
| ‐ reactive mesothelial cells | ||
| Immunostaining | ||
| ‐ AE1/3 | Not performed | + |
| ‐ Calretinin | + | |
| ‐ CD10 | + |
Results from pathologic and cytologic analysis following each surgical procedure
| Test . | Result: index procedure . | Result: repeat procedure . |
|---|---|---|
| Ascites cytology | Benign | Benign |
| Gram stain | Negative | Negative |
| Cultures (fungal, TB) | Negative | Negative |
| Peritoneal biopsy | ‐ Benign fibrous material | Findings similar to index procedure |
| ‐ chronic inflammation | ||
| ‐ reactive mesothelial cells | ||
| Immunostaining | ||
| ‐ AE1/3 | Not performed | + |
| ‐ Calretinin | + | |
| ‐ CD10 | + |
| Test . | Result: index procedure . | Result: repeat procedure . |
|---|---|---|
| Ascites cytology | Benign | Benign |
| Gram stain | Negative | Negative |
| Cultures (fungal, TB) | Negative | Negative |
| Peritoneal biopsy | ‐ Benign fibrous material | Findings similar to index procedure |
| ‐ chronic inflammation | ||
| ‐ reactive mesothelial cells | ||
| Immunostaining | ||
| ‐ AE1/3 | Not performed | + |
| ‐ Calretinin | + | |
| ‐ CD10 | + |
Following surgery, the patient was admitted for monitoring. She recovered well and remained hemodynamically stable without signs of infection or obstruction. Following discharge, she was followed with serial abdominal ultrasound, which demonstrated gradual re-accumulation of ascites over 6 months. Repeat laparoscopy for drainage of ascites was performed, eliciting 2.7 l (https://www.youtube.com/watch?v=uJay5apvuE4&feature=youtu.be). Cytologic evaluation of the fluid again showed benign findings, and pathologic evaluation of additional peritoneal biopsies revealed findings similar to those from the prior surgery. Immunostaining of these specimens showed positive staining for AE1/3, calretinin, and CD10, all consistent with the diagnosis of sclerosing peritonitis. The patient recovered uneventfully following the second procedure. She underwent extensive work-up for rheumatologic and autoimmune diseases, all of which was unrevealing. Specifically, ESR, CRP, ANA, anti-dsDNA, anti-cardiolipin, rheumatoid factor, Lupus anticoagulant, albumin, alkaline phosphatase, complement were all negative or within normal limits. Accordingly, we have counseled her on the symptoms of bowel obstruction, appendicitis and cholecystitis, as well as considerations for future pregnancy and screening colonoscopy, and will continue to monitor with close surveillance, imaging and repeat drainage as necessary.
DISCUSSION
Abdominal cocoon is a rare form of sclerosing peritonitis with no established etiology. It is classically reported to present as bowel obstruction in adolescent and young women, though a recent review found the majority of patients to be men [3]. Most authors agree that surgical resection of the fibrous membrane is the definitive treatment, particularly when patients present with acute bowel obstruction or in patients in whom extra-intestinal organs are encased [3, 4, 6]. In severe cases, bowel resection may be necessary. However, conservative management is also an option, particularly in the earlier stages of disease without intestinal obstruction, abdominal pain or other symptoms. This may consist of observation alone or include medical treatment. Steroids, tamoxifen, colchicine, azathioprine and mycophenolate mofetil have all been reported as effective medical treatments [3, 4, 6, 8, 9].
Our patient presented without symptoms of obstruction despite extensive adhesions sequestering most of her abdominal organs superiorly, and the decision was made to delay surgical resection. Given the extent of fibrosis and lack of organ visibility on laparoscopic examination, surgical take-down of the peritonitis would carry significant risk of organ perforation in a largely asymptomatic patient. This case reinforces the breadth of clinical symptoms that can be seen in abdominal cocoon syndrome, representing a rare example of IESP presenting with massive ascites in the absence of another underlying pathology. Furthermore, it demonstrates the importance of taking a symptom-guided approach to the surgical management of patients with this rare and complex syndrome.
Conflicts of interest
The authors have no conflicts of interest to disclose.
Funding
The authors have no funding or financial relationships to disclose.
Level of evidence: Level V—case report.
References
Author notes
Drs. Rahaman and Popowich are co-senior authors and contributed equally to this article.



