-
PDF
- Split View
-
Views
-
Cite
Cite
Masashi Kawamura, Patricia J Finkbinder, Rohinton J Morris, Reoperative mitral valve replacement via right mini-thoracotomy with ventricular fibrillatory arrest for a patient with severely calcified aortic homograft, Journal of Surgical Case Reports, Volume 2019, Issue 11, November 2019, rjz285, https://doi.org/10.1093/jscr/rjz285
Close - Share Icon Share
Abstract
We successfully performed reoperative mitral valve replacement (MVR) for a patient with a previous extensive cardiac surgery that included aortic homograft replacement for aortic and mitral valve endocarditis complicated with aortic root abscess. The aortic homograft function was well preserved without aortic insufficiency, although the homograft was highly calcified. We used a right mini-thoracotomy approach and ventricular fibrillatory arrest to avoid an aortic cross-clamping. Only minimal dissection was needed to obtain enough exposure to perform the redo MVR. The reduction in invasiveness helped to prevent major injury during the surgery, shortened the cardiopulmonary bypass and operation time, and facilitated the patient’s recovery. Right mini-thoracotomy with ventricular fibrillatory arrest is a viable option for reoperative MVR in patients with previous sternotomy and unclampable aorta.
INTRODUCTION
Right mini-thoracotomy mitral valve surgery has been recently established, and its feasibility, safety and short- and long-term efficacy have been demonstrated to be equivalent to those of the conventional sternotomy approach [1]. The right mini-thoracotomy approach also has advantages in reoperative cardiac surgery by minimizing the dissection and preventing injury of large vessels [2–4]. We herein described a successful case of redo mitral valve replacement (MVR) via right mini-thoracotomy after aortic root replacement with an aortic homograft combined with MVR for aortic and mitral valve-infectious endocarditis complicated with aortic root abscess.
CASE REPORT
The patient was a 56-year-old male with a history of infectious endocarditis of aortic and mitral valves complicated with aortic root abscess. He underwent aortic root replacement with an aortic homograft and MVR with a 27-mm St. Jude mechanical valve. At 7 years after the surgery, he presented to the hospital due to worsening shortness of breath, dark urine and swollen legs. He had hemolysis with lactate dehydrogenase of >1000 U/L. Transesophageal echocardiography demonstrated that the mechanical prosthesis had focal dehiscence at the inferior and inferoseptal margins with severe perivalvular leakage (Fig. 1). The aortic homograft function was well preserved without aortic insufficiency. Right heart catheterization also revealed elevated pulmonary artery and filling pressures (right atrial pressure, 15 mmHg; right ventricular pressure, 88/15 mmHg; pulmonary artery pressure 88/33 (52) mmHg and pulmonary capillary wedge pressure, 33 mmHg). Based on these symptoms and findings, we decided to perform reoperative MVR.
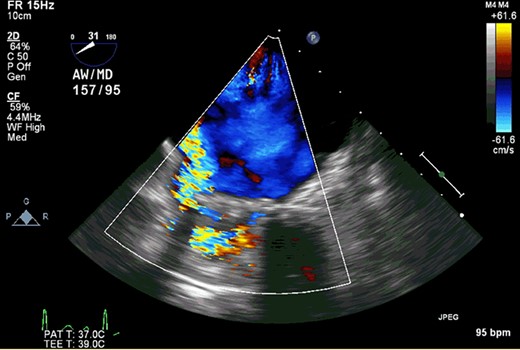
Transesophageal echocardiography demonstrated that the mechanical prosthesis had focal dehiscence with severe perivalvular leakage.
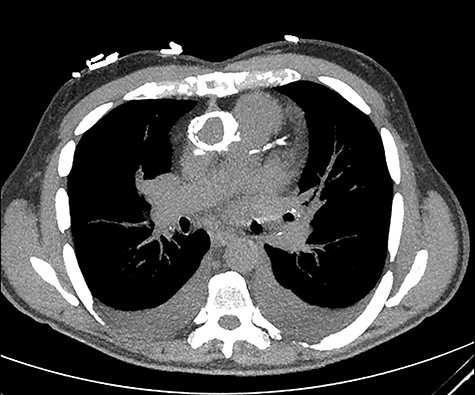
The aortic homograft was circumferentially calcified as shown by a preoperative chest computed tomography scan.
The aortic homograft was circumferentially calcified as shown by a preoperative chest computer tomography scan (Fig. 2). In addition to the extensiveness of the previous operation, severe adhesions around the heart were expected; therefore, a right mini-thoracotomy approach was used. A right anterolateral thoracotomy with a small incision was performed at the fifth intercostal space under single lung ventilation. Cardiopulmonary bypass (CPB) was initiated with the right femoral artery and vein cannulations. Prior to the start of the procedure, an EndoVent™ Pulmonary Catheter (8.3Fr; Edwards Lifesciences, Irvine, CA) was also placed percutaneously through the right internal jugular vein by the anesthesiologist and drained at ~500 ml/min during the CPB. The pericardium was opened, and minimum adhesions were found on the right side of the heart due to the incision of the previous MVR located at the left atrial dome. The right side of the left atrium was dissected, and the right atrium was partially dissected along with the right pulmonary veins. At this point, the patient was cooled down to 30°C for ventricular fibrillation. Once the patient began fibrillating, the left atrium was opened. The mechanical prosthesis was largely detached. The valve was thus duly excised and the annulus was debrided. Interrupted 2–0 Ethibond mattress sutures with pledgets and a 29-mm Mosaic™ mitral valve (Medtronic, Minneapolis, MN) was placed. The left atriotomy was then closed with 2–0 running sutures of 3–0 Prolene around the vent tube that was placed in the left ventricle through the prosthesis. With the patient in the reverse Trendelenburg position and the left side down, the patient was defibrillated. Although the prosthesis was incompetent, the heart was allowed to eject air. After adequate de-airing as confirmed by intraoperative transesophageal echocardiography, the vent tube was removed and the atriotomy was completely closed. CPB was weaned off uneventfully. The ventricular fibrillatory arrest time was 103 min and the CPB time was 150 min.
The patient had no complications during the post-operative course. He recovered well and was discharged 7 days after the surgery.
DISCUSSION
We successfully performed reoperative MVR for a patient with previous extensive cardiac surgery for aortic homograft replacement. The aortic homograft function was well preserved without aortic insufficiency, even though the homograft was highly calcified. We used a right mini-thoracotomy approach and ventricular fibrillatory arrest to avoid aortic cross-clamp so that only minimal dissection was required to obtain enough exposure to perform the reoperative MVR. The reduction in invasiveness helped to prevent major injury during the surgery, shortened the CPB and operation time and facilitated the patient’s quick recovery. A recent report demonstrated that a successful case of reoperative MVR with porcelain aorta and patient coronary artery bypass grafts was performed by right thoracotomy with ventricular fibrillatory arrest [5]. The technique is also useful for MVR with patient coronary artery bypass grafts [3, 5].
The safety of mitral valve surgery through right thoracotomy with fibrillatory arrest or beating heart is still controversial, especially in terms of stroke. A propensity score-matched analysis reported that there were no significant differences in major and minor neurological events between right thoracotomy mitral valve surgery on fibrillatory arrest and conventional median sternotomy [6]. Another report showed that retrograde arterial perfusion was a stronger risk factor for stroke than fibrillatory arrest [7]. On the other hand, using fibrillatory arrest or beating heart in mitral valve surgery presented with an adjusted 3-fold higher risk of stroke, as demonstrated in a Society of Thoracic Surgeons database study [8]. According to the study, air embolism was the most likely mechanism of stroke [8]. It is especially important to pay attention to de-airing, including the patient position and venting methods, when a fibrillatory arrest is used. In the present case, we manipulated the patient’s position and kept the left atrium at the highest position during de-airing. Moreover, the prosthesis was carefully kept incompetent after defibrillation until complete de-airing was obtained.
In conclusion, right mini-thoracotomy with ventricular fibrillatory arrest could be a good option for reoperative MVR in patients with previous sternotomy and unclampable aorta, such as calcified aortic homograft and porcelain aorta, and careful de-airing is needed to prevent neurological complications.
REFERENCES
- aorta
- aortic valve insufficiency
- cardiopulmonary bypass
- cardiac surgery procedures
- abscess
- tissue dissection
- heart ventricle
- surgical procedures, operative
- transplantation, homologous
- surgery specialty
- minithoracotomy
- aortic cross clamping
- supraaortic valve area
- mitral valve endocarditis
- mitral valve replacement, repeat



