-
PDF
- Split View
-
Views
-
Cite
Cite
Ramon Vilallonga, Alejandro Mazarro, María Rita Rodríguez-Luna, Enric Caubet, José Manuel Fort, Manuel Armengol, Xavier Guirao, Massive necrotizing fasciitis: a life threatening entity, Journal of Surgical Case Reports, Volume 2019, Issue 11, November 2019, rjz269, https://doi.org/10.1093/jscr/rjz269
Close - Share Icon Share
Abstract
Necrotizing fasciitis (NF) is a complicated soft tissue infection frequently associated with severe sepsis if an early medical and surgical treatment is not performed. We report two postoperative cases of severe NF after oophorectomy and colorectal resection. Because of the similarity with more benign skin infections at the early steps, clinical suspicion is crucial. Surgical exploration and resection will provide both the diagnosis confirming necrotizing infection of the fascia with vessels and treatment. Also, empirical broad-spectrum antibiotics must be initiated as soon as possible. Regardless of the presence of risk factors, NF is a condition with a high mortality rate and only an expeditious and undelayed treatment may improve the patient’s outcome. Surgical focus control requires wide and repeated resections, and planned reconstructive plastic surgery might be necessary.
Introduction
Necrotizing fasciitis (NF) is a potentially fatal skin infection consisting of liquefaction necrosis of the subcutaneous tissue. Usually, the underlying muscle is preserved.
Necrotizing fascitis usually involve abdominal wall, limbs and perineum. Because of its relatively low incidence, accounting for 3 cases per 100 000 people-year, and the non specific presentation at the very initial steps mimicking benign cellulitis, inadequate and delayed diagnosis may threaten patient's outcome. mostly affects the abdominal wall, extremities and perineum [1, 2], due to its low incidence account of 3 cases per 100 000 person-years and its similarity with cellulitis, this life-threatening condition can be easily mistaken with more benign skin infections, and thus, delay of treatment may compromise patient prognosis [3].
Case Report
A 43-year-old woman with multiple drug allergies (streptomycin, tetracycline and metamizole) required laparoscopic right oophorectomy for incidental ovarian cyst. 48 Hours after the operation, she complained of diffuse left abdominal pain and tachycardia and hypotension were documented and tachycardia and hypotension, requiring vasoactive drugs. Physical exam revealed a diffuse cellulitis on the left abdominal wall that rapidly spread to the vagina a rapid caudal extension to the vagina. CT scan showed pneumoperitoneum and thickening of the abdominal wall in the left flank (Fig. 1). She went to the OR, the exploratory laparotomy demonstrated diffuse fecal peritonitis with two small bowel perforations, requiring primary repair with surgical suturing and without small bowel resection. Empirical antibiotic treatment with meropenem an cliindamycin was started.
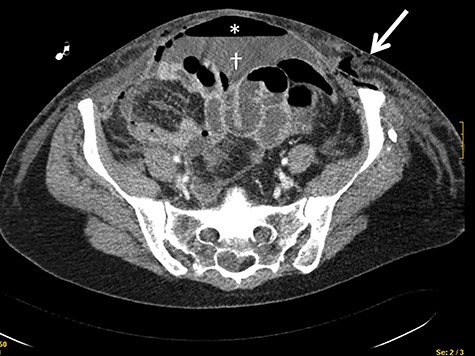
CT scan showing the presence of air in the abdominal wall (arrow) and pneumoperitoneum (asterisks) and intrabdominal liquid (dagger).
On 4th POD, fasciitis progressed to the left flank, thigh and gluteal area gradually extending to the right hemiabdomen (Fig. 2a). Surgery was indicated for extensive fascia resection and microbiologic specimen for culture (Fig. 2b) 48 hours after thigh involvement (Fig. 2c). Samples taken during the repeated surgical debridement revealed the presence of Candida albicans intrabdominal sample and antifungeal therapy was started. The patient was uneventfully recovered One month after the onset of symptoms (Table 1), reconstructive surgery was scheduled for skin grafting (Fig. 2d). Shortly after the reconstructive procedure patient presented a massive upper gastrointestinal tract bleeding requiring emerging gastroscopy. Hemodynamic instability led to cardiac arrest, requiring 20 minutes of advanced CPR and 48 hours later death due to multiorgan failure.
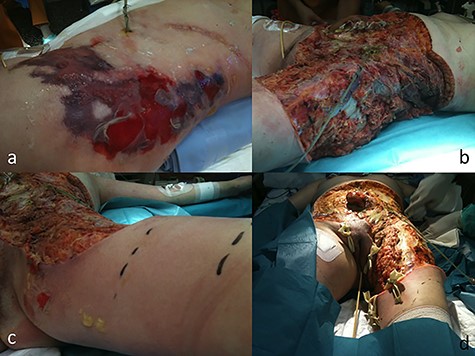
Case 1 (a) Skin lesions observed in the ICU before the surgery. (b) Final image after debridement. At this stage of the procedure, it was not technically possible to close the patient’s abdominal wall. (c) Left leg extension after 24 hours after the first procedure. (d) Abdominal wall and leg once in the plastic and reconstructive surgery before the skin grafts.
| . | L (10E12/L) . | Hb (g/dL) . | Cr (mg/dL) . | CRP (mg/dL) . | T (°C) . | SBP (mmHg) . | DBP (mmHg) . | HR (bpm) . |
|---|---|---|---|---|---|---|---|---|
| 1st POD | 8.6 | 12.2 | 1.83 | 40 | 37 | 89 | 60 | 95 |
| 2nd POD | 0.9 | 10 | 1.34 | 28 | 37 | 96 | 64 | 116 |
| 4th POD | 13.6 | 9.7 | 1.15 | 19 | ||||
| 6th POD | 28 | 10 | 0.8 | 20 | 39.3 | 123 | 81 | 125 |
| 10th POD | 21.4 | 11 | 0.38 | 6 | ||||
| 18th POD | 11.9 | 10 | 0.22 | 14 | 37.5 | 114 | 70 | 100 |
| 30th POD | 8.4 | 11 | 0.33 | 8,5 | 36.5 | 140 | 85 | 99 |
| 37th POD | 5.6 | 11 | 0.17 | |||||
| 47th POD | 13.4 | 11 | 0.63 | 37.9 |
| . | L (10E12/L) . | Hb (g/dL) . | Cr (mg/dL) . | CRP (mg/dL) . | T (°C) . | SBP (mmHg) . | DBP (mmHg) . | HR (bpm) . |
|---|---|---|---|---|---|---|---|---|
| 1st POD | 8.6 | 12.2 | 1.83 | 40 | 37 | 89 | 60 | 95 |
| 2nd POD | 0.9 | 10 | 1.34 | 28 | 37 | 96 | 64 | 116 |
| 4th POD | 13.6 | 9.7 | 1.15 | 19 | ||||
| 6th POD | 28 | 10 | 0.8 | 20 | 39.3 | 123 | 81 | 125 |
| 10th POD | 21.4 | 11 | 0.38 | 6 | ||||
| 18th POD | 11.9 | 10 | 0.22 | 14 | 37.5 | 114 | 70 | 100 |
| 30th POD | 8.4 | 11 | 0.33 | 8,5 | 36.5 | 140 | 85 | 99 |
| 37th POD | 5.6 | 11 | 0.17 | |||||
| 47th POD | 13.4 | 11 | 0.63 | 37.9 |
L: Leukocytes; Hb: Hemoglobin; Cr: Creatinine; CRP: C-reactive protein; T: Temperature; SBP: Systolic blood pressure; DBP: Diastolic blood pressure; HR: Heart rate.
| . | L (10E12/L) . | Hb (g/dL) . | Cr (mg/dL) . | CRP (mg/dL) . | T (°C) . | SBP (mmHg) . | DBP (mmHg) . | HR (bpm) . |
|---|---|---|---|---|---|---|---|---|
| 1st POD | 8.6 | 12.2 | 1.83 | 40 | 37 | 89 | 60 | 95 |
| 2nd POD | 0.9 | 10 | 1.34 | 28 | 37 | 96 | 64 | 116 |
| 4th POD | 13.6 | 9.7 | 1.15 | 19 | ||||
| 6th POD | 28 | 10 | 0.8 | 20 | 39.3 | 123 | 81 | 125 |
| 10th POD | 21.4 | 11 | 0.38 | 6 | ||||
| 18th POD | 11.9 | 10 | 0.22 | 14 | 37.5 | 114 | 70 | 100 |
| 30th POD | 8.4 | 11 | 0.33 | 8,5 | 36.5 | 140 | 85 | 99 |
| 37th POD | 5.6 | 11 | 0.17 | |||||
| 47th POD | 13.4 | 11 | 0.63 | 37.9 |
| . | L (10E12/L) . | Hb (g/dL) . | Cr (mg/dL) . | CRP (mg/dL) . | T (°C) . | SBP (mmHg) . | DBP (mmHg) . | HR (bpm) . |
|---|---|---|---|---|---|---|---|---|
| 1st POD | 8.6 | 12.2 | 1.83 | 40 | 37 | 89 | 60 | 95 |
| 2nd POD | 0.9 | 10 | 1.34 | 28 | 37 | 96 | 64 | 116 |
| 4th POD | 13.6 | 9.7 | 1.15 | 19 | ||||
| 6th POD | 28 | 10 | 0.8 | 20 | 39.3 | 123 | 81 | 125 |
| 10th POD | 21.4 | 11 | 0.38 | 6 | ||||
| 18th POD | 11.9 | 10 | 0.22 | 14 | 37.5 | 114 | 70 | 100 |
| 30th POD | 8.4 | 11 | 0.33 | 8,5 | 36.5 | 140 | 85 | 99 |
| 37th POD | 5.6 | 11 | 0.17 | |||||
| 47th POD | 13.4 | 11 | 0.63 | 37.9 |
L: Leukocytes; Hb: Hemoglobin; Cr: Creatinine; CRP: C-reactive protein; T: Temperature; SBP: Systolic blood pressure; DBP: Diastolic blood pressure; HR: Heart rate.
Second case: a 77 year-old man with hepatitis C virus infection was admitted for an scheduled left hemicolectomy in the setting of colon cancer at the splenic flexure. On postoperative day 4 after the surgery, skin lesions appeared in both flanks and he was diagnosed with an allergic reaction. He received systemic corticosteroids and antihistaminic without clinical improvement. In the following 24 hours, his conscious level decreased gradually, he presented fever and cardiac arrest and recovered after 15 minutes of CPR. Sepsis was suspected and Meropenem was empirically started. CT scan was performed without any relevant findings. Skin erythema progressed, affecting both flanks and thighs (Fig. 3); blisters and ecchymosis appeared after few hours and surgical exploration documented an extended fascial necrosis. The family didn’t allow more invasive procedures and the patient died after 6 days.
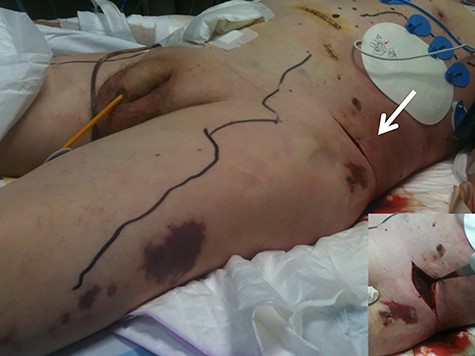
Case 2. Image showing the extension of the fasciitis along the abdomen and legs. Many incisions were made over the patient’s abdominal wall, revealing extensive necrotic tissue (arrow).
Discussion
NF is an extremely severe soft tissue infection involving the deep layers of skin and requires aggressive treatment. It usually occurs in patients with comorbidity such as immunosuppression, drug abuse and diabetes, although it can also occur in healthy individuals, as in the first case reported.
As early skin manifestations may mimicks a benign cellulitis, early diagnosis is often difficult and requires a high index of suspicion. Clinical findings include erythema, swelling, numbness and tenderness of the affected area and occasionally blisters. It is accompanied by progressive and disproportioned pain and signs of systemic sepsis. Crepitus may be found in case of infection by gas-forming organisms. Nevertheless, such specific signs use to appear when NF is completely consolidated and this evident portray does not help for an early diagnosis and treatment.
The physiopathology of NF is related to an injury that allows bacteria penetration into the subcutaneous space although the entry point is not always identifiable [4]. Microbiological studies reveal a large range of pathogens; the majority of cases are produced by a polymicrobial infection. Angoules et al. in a systematic review stated that Staphylococcus aureus, group A Streptococcus pyogenes and Streptococcus viridans are the three most common bacteria found [5].
Based on wound microbiology, Morgan et al. defined four types of NF; type I (70–80%) is produced by a polymicrobial infection, type II (10–20%) responds to a group A hemolytic Streptococcus, type III is caused by Gram-negative marine-related organisms (Vibrio spp) and type IV is produced by a fungal infection [6].
Image tools to complement diagnosis include magnetic resonance imaging (MRI), computed tomography (CT), ultrasonography (US) and plain radiography. CT findings encompasses fat stranding and fascial thickening with fluid or gas collections over the fascial planes. MRI is useful to differentiate NF from cellulitis and is 100% sensitive and 86% specific in the diagnosis, although an overestimation extent of the infection has been described. Studies have showed that the use of ultrasound (US) for early diagnosis, US report a sensibility and specific of 88.2% and 93.3% respectively, findings include diffuse subcutaneous thickening, fluid layer of >4 mm along the deep fascia and gas in the soft tissues, which is pathognomonic [7, 8].
Wong et al. designed the LRINEC score, which uses basic laboratory parameters such as PPV and NPV of 92% and 96%, respectively. A score of ≥6 should raise the suspicion and a score of ≥8 is strongly predictive. Despite medical advances, NF is a severe condition with a high mortality rate, ranging from 9.3 to 76% (20). Death usually is produced by septic shock and multiorgan failure [9].
Early antibiotic therapy must be established against a wide range of microorganisms. Hunter et al. suggest the combination of a carbapenem with clindamycin [10]. Otherwise, If cultures demonstrate sensitive Streptococcus pyogenes, there is a general agreement that a combination of penicillin G and clindamycin (high doses) is the prefered option.
Surgery is the crown jewel of NF treatment. It is imperative to perform an aggressive exploration and excision of all the necrotic and devitalized area, with margins of 5–10 mm. Multiple operations are often necessary. Although some studies did not find significant correlation between early surgical debridement and clinical outcomes, it is globally accepted that any delay in diagnosis and surgery is associated with a clear increase in mortality [11]. The rule of thumb is if deep soft tissue is suspected based on epidemiological, clinical and seminal local inflammatory data, is much better to perform surgical exploration than wait and see.
Wound management includes vacuum-assisted closure therapy and hyperbaric oxygen therapy especially when anaerobic clostridial organisms are detected; immunoglobulin has also been used.
Nowadays NF remains a high mortality entity; only an expeditious and undelayed treatment may improve prognosis. Surgical focus control requires wide and repeated resections, and planned reconstructive plastic surgery might be necessary. These cases highlight that diagnosis of NF is challenging and surgeons must be aware of the importance of rapid diagnosis and treatment to prevent mortality. Surgery remains the principal treatment; debridement must be performed as early as possible and must not be delayed by diagnostic methods; antibiotic therapy including broad spectrum and multidisciplinary treatment must be performed to prevent mortality.
ACKNOWLEDGEMENT
The authors want to thank the Dr. Vilallonga Foundation for the financial support in the preparation of this article (http://www.fundacioramonvilallonga.org).
CONFLICTS OF INTEREST
There are no conflicts of interest.
References
Author notes
Fellowship in Minimal Invasive Surgery



