-
PDF
- Split View
-
Views
-
Cite
Cite
Erica Hill, Amelia Merrill, Soheila Korourian, Gwendolyn Bryant-Smith, Ronda Henry-Tillman, Daniela Ochoa, Silicone breast implant associated fibromatosis, Journal of Surgical Case Reports, Volume 2018, Issue 9, September 2018, rjy249, https://doi.org/10.1093/jscr/rjy249
Close - Share Icon Share
Abstract
Extra-abdominal desmoid tumors, also known as aggressive or deep fibromatosis, are uncommon soft tissue tumors that rarely involve the breast. Although the exact etiology is unknown, the development of these tumors has been correlated with sites of previous trauma, surgery or in association with familial adenomatous polyposis. Clinically, breast fibromatosis is often mistaken for carcinoma but lacks metastatic potential. It is locally aggressive with high rates of recurrence. The treatment is primarily wide local excision with negative margins. Adjuvant treatments have been suggested and include radiotherapy, chemotherapy and hormonal therapy, however, there are no evidence-based treatment protocols to support their use. Here, we describe a case of fibromatosis that developed within the capsule around a silicone breast implant treated with surgical excision alone. The patient remains recurrence free at 3 months post-operative magnetic resonance imaging.
INTRODUCTION
Extra-abdominal desmoid tumors, also known as deep fibromatosis (DF), are uncommon soft tissue tumors that rarely involve the breast. They account for 0.3% of all solid neoplasms and <0.2% of breast tumors [1, 2]. The most common sites for DF are the limbs (50%), trunk (43%), or head and neck (7%) [3]. The exact etiology is unknown but a majority of DF occur sporadically with 85% of cases having a somatic mutation in the β-catenin-activating gene [2–4]. Strong correlations have also been made between their development and sites of previous trauma or surgery, hormonal influences, or in association with familial adenomatous polyposis [1–7]. A majority of breast DF occur in women, and desmoid tumors in general are more common in females than males with a 2:1 gender ratio [1, 2]. The hypothesis that estrogen has an influence on the incidence of DF is supported by the preponderance of female cases and the growth or development of DF during pregnancy [1, 8].
The diagnosis of breast DF can be difficult to determine preoperatively, and is often mistaken for carcinoma due to appearance on imaging and clinical presentation [1]. Breast fibromatosis lack metastatic potential but are locally aggressive with high rates of recurrence despite adequate excision [1, 2, 5, 6]. They can develop from fibroblasts or myofibroblasts in the breast parenchyma, the musculoaponeurotic layer of the pectoralis muscle, or in association with the capsule that forms around a breast implant [2, 5, 6]. Here, we describe a case of fibromatosis that developed within the capsule around a silicone breast implant.
CASE REPORT
A 34-year-old female presented to our institution’s outpatient breast oncology clinic with a palpable left breast mass. She underwent bilateral breast augmentation with sub-muscular silicone implants at an outside facility 3 years previously. She first noticed a soft, mobile mass 6 months prior to presentation that had become more firm and increased in size over time. She also reported being involved in a motor vehicle accident 3 months before the onset of symptoms, where her chest was impacted by the steering wheel.
On physical exam, there was an asymmetry to the projection of her breasts with the inferior pole of the left breast higher than the right. There was a firm, 7 × 4 cm2 mass of the left breast separate from the breast implant at 11 o'clock 9 cm from the nipple (Fig. 1). The right breast was benign with no masses appreciated.

Clinical image demonstrates soft tissue mass of the left breast in the 11 o'clock position, 9 cm from the nipple, clinically measuring 6.4 × 3.5 cm2.
Ultrasound revealed a well-defined, heterogeneously, hypoechoic mass abutting the superior medial portion of the intact implant (Fig. 2). The lateral aspect of the mass demonstrated a characteristic ‘fascial-tail’ sign described by Huang et al. [8] as the linear extension of the tumor along the fascial planes diagnostic of fibromatosis. The mass extended into the intercostal space posteriorly. There was evidence of increased vascularity. The axilla was evaluated and showed no suspicious axillary lymphadenopathy. Magnetic resonance imaging (MRI) without contrast demonstrated an 8.1 × 4.7 × 7.1 cm3 slightly lobulated mass abutting the un-ruptured silicone breast implant (Fig. 3). The mass extended into the anterior intercostal space between the second and third ribs, with no definitive involvement of the ribs. On T1 weighted sequences, the lesion was heterogeneous and had low signal; signal intensity was hyperintense on gradient-echo imaging. The pectoralis muscle was seen extending over the mass but without definitive involvement.
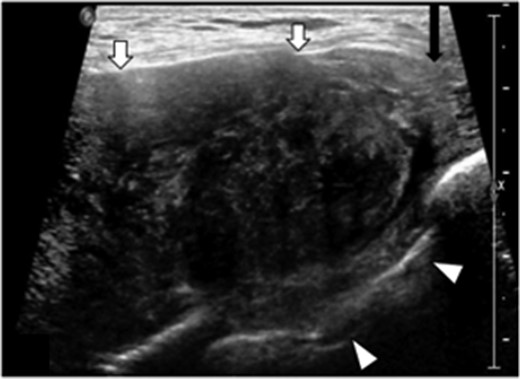
Transverse targeted ultrasound image of palpable breast mass show a large heterogeneously, hypoechoic mass with internal vascularity, well defined (white arrows). Mass extends between the ribs into the intercostal space (arrowheads). Beak like appearance at the lateral margin (characteristic ‘fascial tail’ sign, black arrow).
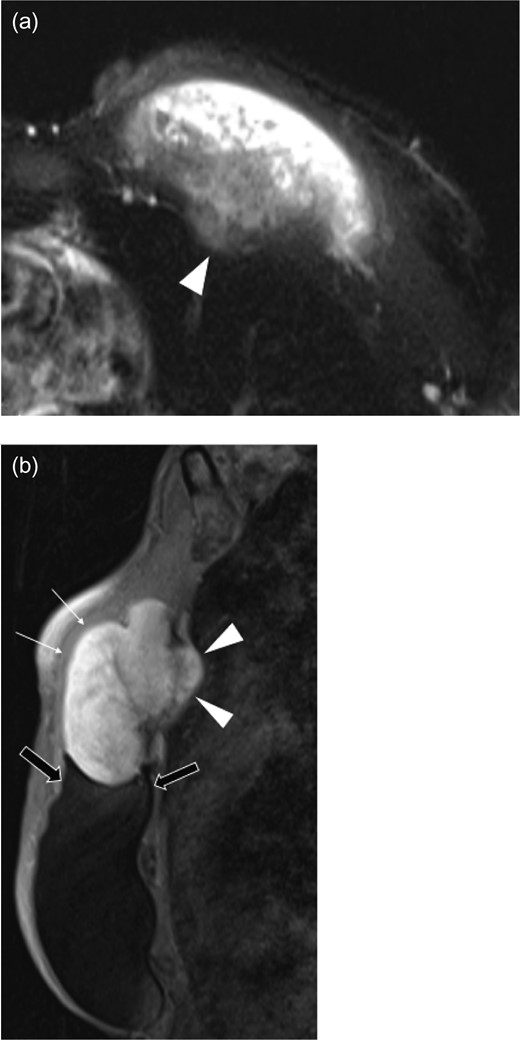
Axial T2 weighted half-fournier acquired single-shot turbo spin echo (HASTE) (a), and sagittal fat suppression (b) images of left breast mass. The pectoralis major muscle (white arrows) overlies the large mass that extends into the intercostal space (arrowheads) between the second and third ribs. The lobulated mass is in direct contact with the breast implant (black arrows).
A core needle biopsy (CNB) was obtained and reported as a spindle cell lesion (Fig. 4). No cytologic atypia or mitotic figures were seen. Diffuse nuclear positivity with β-catenin was noted. The cells did not express CD34. Fibromatosis was considered high on the differential diagnosis, as well as other sarcomas and spindle cell carcinoma.
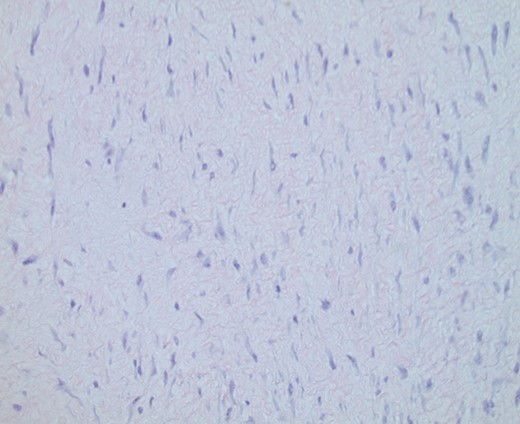
Original CNB demonstrating spindle cell lesion. Hematoxylin and eosin (H&E) ×100 magnification.
The patient was taken for wide local excision of the lesion (Fig. 5). The lesion was excised in its entirety without violation. It was noted to be located within the capsule of the left breast implant and attached to the chest wall and third rib posteriorly, however, able to be dissected free from these underlying structures. Grossly, the specimen was a tan-white, fibrous, solid mass that measured 11.0 × 8.0 × 4.5 cm3. The silicone breast implant was left in place. Microscopic analysis revealed long fascicles of spindle cells with no or minimal cytologic atypia and no mitotic figures (Fig. 6). Focal prominent stromal collagen was demonstrated. The diagnosis of fibromatosis was supported by positive nuclear staining with β-catenin, which is seen in 70–80% of cases [6]. Our tumor did not show any p63 positivity, which would have supported the diagnosis of spindle cell carcinoma. Negative margins were obtained. This case was discussed at the institutional multidisciplinary tumor conference with recommendation for close surveillance but no additional adjuvant treatment. A follow up breast MRI performed at 3 months post-operatively demonstrated no recurrent mass or abnormal enhancement. At 8 months she remains recurrence free.
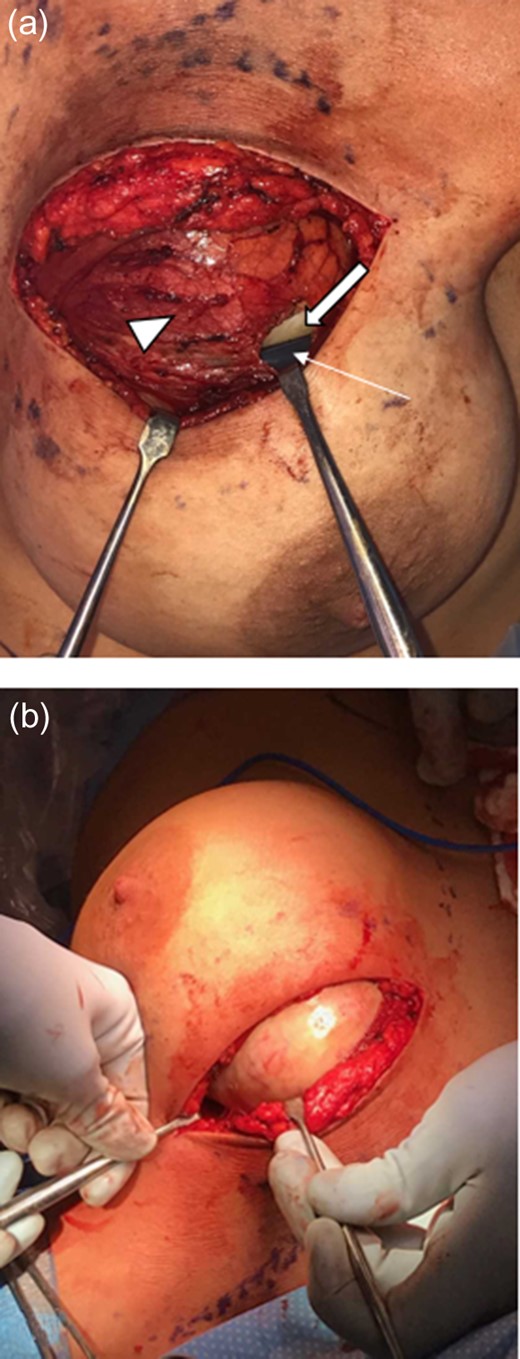
Intraoperative images. (a) Capsule (arrow head) surrounds the mass (block arrow) and adjacent implant (arrow). (b) Capsule is retracted revealing white mass.
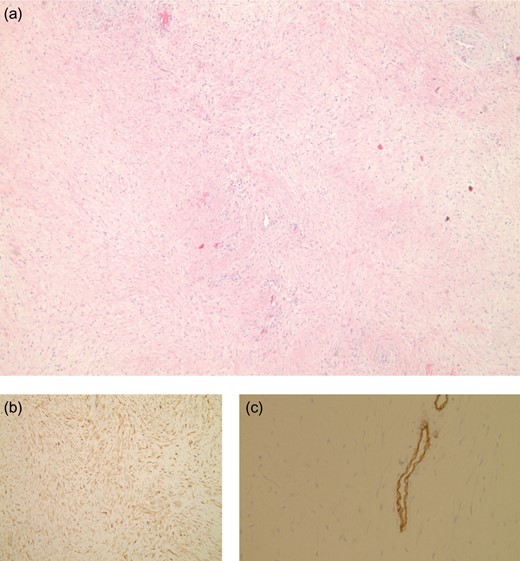
Resected mass at ×40 magnification (a) shows long fascicles of spindle cells. Nuclear positivity with β-catenin (b), and immuno-stain for CD34 highlights the blood vessels (c), the neoplastic cells are CD34 negative.
DISCUSSION
Fibromatosis associated with breast implants is rare. Reviews of previously reported cases found that the mean interval to tumor development from the time of implant placement was ~3 years [4, 5]. More cases involve silicone implants versus saline, however, it is unclear as to whether there is a predilection for formation with silicone implants or if this reflects the higher frequency of silicone implant use [5].
Primary treatment for breast fibromatosis is wide local excision that can involve resection of a portion of the chest wall to obtain negative margins. Adjuvant modalities such as radiotherapy, chemotherapy and hormonal therapy have been suggested, however, there are no evidence-based treatment protocols to support their use [4, 5, 7]. In a 28 patient case series reviewing surgical management of desmoid tumors of the breast at Memorial Sloan Kettering Cancer Center, a 29% recurrence rate was observed [1]. Risk factors for recurrence include positive margins after excision, large tumor size, young age [1, 5].
Although rare, the diagnosis fibromatosis should be considered in the setting of breast augmentation. Optimal management should include the involvement of a multidisciplinary team, particularly in cases of margin positivity, recurrence or unresectability.
DISCLOSURE
The authors report no disclosures or conflict of interest
FUNDING
This research was supported by a grant from the Arkansas Breast Cancer Research Programs.
REFERENCES
- magnetic resonance imaging
- radiation therapy
- carcinoma
- chemotherapy regimen
- familial adenomatous polyposis
- immunologic adjuvants
- pharmaceutical adjuvants
- fibromatosis, aggressive
- soft tissue neoplasms
- breast
- neoplasms
- endocrine therapy
- silicone breast implants
- fibromatosis
- excision
- trauma surgery
- causality
- extra-abdominal fibromatosis
- evidence-based treatment
- wide local excision



