-
PDF
- Split View
-
Views
-
Cite
Cite
German Mínguez Ruiz, Luis J García Florez, R Dario Arias Pacheco, Isabel García Bear, Virginia Ramos Pérez, Gerardo Pire Abaitua, Post-nephrectomy diaphragmatic hernia. Increase suspicion and decrease morbi-mortality: two cases report, Journal of Surgical Case Reports, Volume 2018, Issue 8, August 2018, rjy224, https://doi.org/10.1093/jscr/rjy224
Close - Share Icon Share
Abstract
Post-nephrectomy diaphragmatic hernia is an extremely rare condition. The symptoms may be acute or latent and will depend on the herniated organ, which makes it difficult to suspect. Therefore, it is necessary to know about this type of iatrogenic hernia to avoid a delay in diagnosis. A radiological confirmation with computed tomography and early surgical treatment greatly decreased the morbidity and mortality. We report two cases: a 76-year-old male, who underwent a right nephrectomy 18 days prior due to a renal carcinoma; and a 59-year-old woman, who underwent the procedure 4 years prior due to left renal atrophy.
INTRODUCTION
The most common among acquired diaphragmatic hernias are those of traumatic origin [1]. An iatrogenic diaphragmatic hernia is rarer and is defined as an acquired defect of the diaphragm, following a thoracic or abdominal surgical procedure during which a lesion occurs that may go unnoticed. The subject of this article is the unusual presentation of two cases of diaphragmatic hernia in relation to prior nephrectomy.
CASE 1
This is a 76-year-old male who came to the Emergency Department with intense periumbilical abdominal pain radiating to the back and vomiting. The patient underwent a right laparoscopic radical nephrectomy 18 days before. There were no complications during the procedure and post-operative period. Physical examination showed right basal hypoventilation with abdominal pain and tenderness in the right upper quadrant. A CT scan showed an orifice in the right diaphragm, through which intestinal loops passed into the thorax, which were dilated and with signs of ischemia (Fig. 1), image not present in the pre-operative CT. The patient underwent emergency surgery, via a right subcostal laparotomy. A diaphragmatic hernia ~6 cm in size was found in the right posterior costophrenic angle with a nonviable loop of ileum, reduced and resected after mobilization of the right hepatic lobe. The orifice was closed with interrupted tension-free stitches. Post-operative recovery was uneventful. One year after the surgery, the patient is asymptomatic and shows no signs of hernia recurrence.
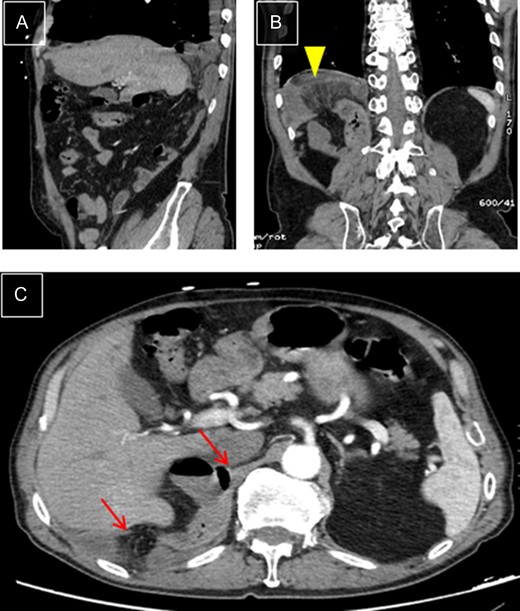
Right diaphragmatic hernia. (A) Thoracic-abdominal CT angiography, sagittal cut, showing right posterior location. (B) Coronal cut: Ileal loop in supradiaphragmatic position (arrowhead). (C) Transverse cut: edges of hernial ring (arrows).
CASE 2
This is a 59-year-old woman. She underwent a left laparoscopic nephrectomy 4 years ago. She was admitted into the Emergency Department with anterior chest pain irradiated to the left shoulder changing with respiratory. The oxygen saturation was 90% and auscultation revealed a lack of breath sounds in the lower half of the left side of the chest. The abdomen was soft and not tender. The CT scan showed a large left diaphragmatic hernia, with intra-thoracic dilated stomach, spleen and descending colon, that led to the collapse of a large part of the left lung, with shifting of the mediastinum towards the right side of the chest (Fig. 2), not present in the pre-operative CT. An emergency laparotomy was performed, identifying a major left diaphragmatic defect, with herniation of the structures previously described that were reduced and performing a tension-free suture with interrupted stitches. There were no complications during the post-operative period. One year later, the patient is in good condition with no signs of recurrence.
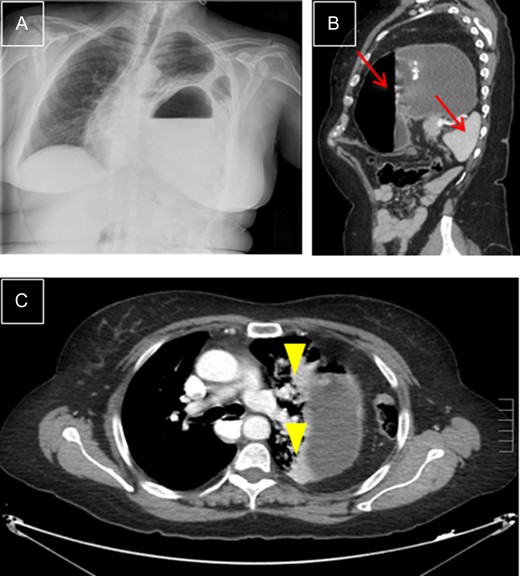
Left diaphragmatic hernia. (A) Chest X-ray with visceral herniation in the chest and mediastinal shift. (B) CT, sagittal view with dilated stomach and spleen in left side of chest (arrows). (C) CT, transverse view: compressed lung parenchyma with atelectasis due to gastric compression (arrowheads).
DISCUSSION
There is a low incidence of iatrogenic diaphragmatic hernias. In a comprehensive review of the literature, we have found 37 cases and only 10 were post-nephrectomy (Table 1). The mechanism by which they seem to occur is a direct or thermal lesion of the diaphragm fibers that creates a point of weakness or a punctiform perforation that goes unnoticed [2]. During the surgery, the patient is intubated with positive pressures in the chest, so it is easier for the orifice to remain collapsed. But in the post-operative period, the pressure gradient between the chest and the abdomen makes the area of weakness open and the small orifice dilates over time, until it allows migration of the abdominal organs into the chest [3, 4]. Several factors can promote this mechanism: diaphragm with weak walls, increase in abdominal pressure or post-operative adhesions that can exert traction at the diaphragmatic level [2].
| Reference . | Year . | Age . | Hernia location . | Cause of nephrectomy . | Time first Surg/Diag* . | Herniated organ . | Approach . | Diaphragmatic closed . |
|---|---|---|---|---|---|---|---|---|
| Axon (3) | 1995 | 36 | Left | Calculus + abscess | 6 months | Stomach | Torax | Suture |
| Peterli (3) | 1996 | – | Right | – | 5 years | – | Abdomen | – |
| Yamashita (3) | 2000 | 56 | Left | Abscess | 7 months | Stomach | – | Mesh |
| Rompen (11) | 2005 | 73 | Right | Renal cancer | 6 years | Colon, small bowel | Abdomen | Suture |
| De Meijer (3) | 2008 | 47 | Left | Renal cancer | <1 day | Stomach, spleen, colon, omentum | Torax | Mesh |
| Ishibe (10) | 2009 | 81 | Right | Renal cancer | 3 years | Colon, right liver | Torax + abdomen | Mesh |
| Frohme (6) | 2009 | 69 | Left | Abscess | Days | Stomach, small bowel | No surgery | – |
| Fitzgerald (5) | 2013 | 35 | Left | Polycystic | 3 years | Stomach | Abdomen | Suture |
| Fitzgerald (5) | 2013 | 67 | Left | Renal cancer | 13 months | Stomach | Abdomen | Suture |
| Guglielmo (12) | 2015 | 37 | Left | Renal cancer | – | – | Torax | – |
| Current study | 2016 | 76 | Right | Renal cancer | 18 days | Small bowel | Abdomen | Suture |
| Current study | 2016 | 59 | Left | Atrophy | 4 years | Stomach, spleen | Abdomen | Suture |
| Reference . | Year . | Age . | Hernia location . | Cause of nephrectomy . | Time first Surg/Diag* . | Herniated organ . | Approach . | Diaphragmatic closed . |
|---|---|---|---|---|---|---|---|---|
| Axon (3) | 1995 | 36 | Left | Calculus + abscess | 6 months | Stomach | Torax | Suture |
| Peterli (3) | 1996 | – | Right | – | 5 years | – | Abdomen | – |
| Yamashita (3) | 2000 | 56 | Left | Abscess | 7 months | Stomach | – | Mesh |
| Rompen (11) | 2005 | 73 | Right | Renal cancer | 6 years | Colon, small bowel | Abdomen | Suture |
| De Meijer (3) | 2008 | 47 | Left | Renal cancer | <1 day | Stomach, spleen, colon, omentum | Torax | Mesh |
| Ishibe (10) | 2009 | 81 | Right | Renal cancer | 3 years | Colon, right liver | Torax + abdomen | Mesh |
| Frohme (6) | 2009 | 69 | Left | Abscess | Days | Stomach, small bowel | No surgery | – |
| Fitzgerald (5) | 2013 | 35 | Left | Polycystic | 3 years | Stomach | Abdomen | Suture |
| Fitzgerald (5) | 2013 | 67 | Left | Renal cancer | 13 months | Stomach | Abdomen | Suture |
| Guglielmo (12) | 2015 | 37 | Left | Renal cancer | – | – | Torax | – |
| Current study | 2016 | 76 | Right | Renal cancer | 18 days | Small bowel | Abdomen | Suture |
| Current study | 2016 | 59 | Left | Atrophy | 4 years | Stomach, spleen | Abdomen | Suture |
*Time between first surgery and diagnosis of diaphragmatic hernia.
| Reference . | Year . | Age . | Hernia location . | Cause of nephrectomy . | Time first Surg/Diag* . | Herniated organ . | Approach . | Diaphragmatic closed . |
|---|---|---|---|---|---|---|---|---|
| Axon (3) | 1995 | 36 | Left | Calculus + abscess | 6 months | Stomach | Torax | Suture |
| Peterli (3) | 1996 | – | Right | – | 5 years | – | Abdomen | – |
| Yamashita (3) | 2000 | 56 | Left | Abscess | 7 months | Stomach | – | Mesh |
| Rompen (11) | 2005 | 73 | Right | Renal cancer | 6 years | Colon, small bowel | Abdomen | Suture |
| De Meijer (3) | 2008 | 47 | Left | Renal cancer | <1 day | Stomach, spleen, colon, omentum | Torax | Mesh |
| Ishibe (10) | 2009 | 81 | Right | Renal cancer | 3 years | Colon, right liver | Torax + abdomen | Mesh |
| Frohme (6) | 2009 | 69 | Left | Abscess | Days | Stomach, small bowel | No surgery | – |
| Fitzgerald (5) | 2013 | 35 | Left | Polycystic | 3 years | Stomach | Abdomen | Suture |
| Fitzgerald (5) | 2013 | 67 | Left | Renal cancer | 13 months | Stomach | Abdomen | Suture |
| Guglielmo (12) | 2015 | 37 | Left | Renal cancer | – | – | Torax | – |
| Current study | 2016 | 76 | Right | Renal cancer | 18 days | Small bowel | Abdomen | Suture |
| Current study | 2016 | 59 | Left | Atrophy | 4 years | Stomach, spleen | Abdomen | Suture |
| Reference . | Year . | Age . | Hernia location . | Cause of nephrectomy . | Time first Surg/Diag* . | Herniated organ . | Approach . | Diaphragmatic closed . |
|---|---|---|---|---|---|---|---|---|
| Axon (3) | 1995 | 36 | Left | Calculus + abscess | 6 months | Stomach | Torax | Suture |
| Peterli (3) | 1996 | – | Right | – | 5 years | – | Abdomen | – |
| Yamashita (3) | 2000 | 56 | Left | Abscess | 7 months | Stomach | – | Mesh |
| Rompen (11) | 2005 | 73 | Right | Renal cancer | 6 years | Colon, small bowel | Abdomen | Suture |
| De Meijer (3) | 2008 | 47 | Left | Renal cancer | <1 day | Stomach, spleen, colon, omentum | Torax | Mesh |
| Ishibe (10) | 2009 | 81 | Right | Renal cancer | 3 years | Colon, right liver | Torax + abdomen | Mesh |
| Frohme (6) | 2009 | 69 | Left | Abscess | Days | Stomach, small bowel | No surgery | – |
| Fitzgerald (5) | 2013 | 35 | Left | Polycystic | 3 years | Stomach | Abdomen | Suture |
| Fitzgerald (5) | 2013 | 67 | Left | Renal cancer | 13 months | Stomach | Abdomen | Suture |
| Guglielmo (12) | 2015 | 37 | Left | Renal cancer | – | – | Torax | – |
| Current study | 2016 | 76 | Right | Renal cancer | 18 days | Small bowel | Abdomen | Suture |
| Current study | 2016 | 59 | Left | Atrophy | 4 years | Stomach, spleen | Abdomen | Suture |
*Time between first surgery and diagnosis of diaphragmatic hernia.
The majority are located on the left side, probably due to the ‘protective’ effect that the liver exerts over the diaphragm (hence the special rarity of our first case), and can develop from the first day to years after the procedure [5]. In post-nephrectomy hernias, patients may present with latent symptoms or it may appear as a casual finding in an imaging test, which means that this pathology can be underdiagnosed [6]. In cases of acute symptoms, the most typical symptom is epigastric and/or chest pain, which is at times associated with dyspnea, reduced breath sounds and even appearance of bowel sounds in the chest. Obstructive symptoms could appear when the hernia contains a hollow organ [4, 5, 7]. Delayed diagnosis can lead to a life-threatening outcome with ischemia or perforation of the herniated organs [5]. To confirm the diagnosis, chest X-rays can show irregularity in the diaphragmatic contour, organ herniation in the chest, pleural effusion and/or mediastinal shift to the other side. However, 50% of them will have a false negative result [7, 8]. The most sensitive test for diagnosis is the chest-abdominal CT scan [1], which shows the diaphragmatic discontinuity, the herniated content and if there are signs of obstruction or ischemia. It also allows a plan to be made before surgery [9].
Surgical treatment is of choice. In the emergency onset, diagnostic delay means an increase in morbidity and mortality. There is no consensus about the better approach: some authors recommend chest approach, which has the advantage of avoiding intra-abdominal adhesions, reduction of the content and good visibility of the defect [3]. However, the need for mechanical ventilation and the hospital stay are increased [7]. Others prefer laparotomy, especially if there is a suspicion of damage of the herniated content, although reduction of this content can be more complex. This access could be better for both identification and repair of organ lesions [3, 9]. In complex cases, the better approach will be combined. Laparoscopy use to be safe and effective with reduced hospital stay. Our recommendation is that surgeon’s choice will depend on his own experience. Correction of the hernia orifice can be done in most cases with suturing the defect; non-absorbable tension-free sutures are generally used to re-establish the anatomy of the diaphragm. In large orifices where the repair cannot be ensured without tension, a prosthetic mesh is recommended [1].
CONCLUSION
Post-nephrectomy diaphragmatic hernia is a very uncommon condition. Primary prevention, with careful management of instruments near the diaphragm, as well as a final check thereof, both in open surgery and laparoscopy, is the best way to avoid the morbidity and mortality it could cause [8]. We must think in this possible complication in our differential diagnosis to avoid delayed treatment.
CONFLICT OF INTEREST
The authors have no conflict of interests.



