-
PDF
- Split View
-
Views
-
Cite
Cite
Yuki Owada-Ozaki, Yuki Matsumura, Hiroyuki Suzuki, VV-ECMO during subsequent segmentectomy after right pneumonectomy, Journal of Surgical Case Reports, Volume 2018, Issue 8, August 2018, rjy213, https://doi.org/10.1093/jscr/rjy213
Close - Share Icon Share
Abstract
Several reports have described subsequent pulmonary surgery after pneumonectomy. We herein report the case of an 82-year-old woman with metachronous multiple lung cancer who had undergone surgery for adenocarcinoma of the right upper lobe 17 years earlier. Completion pneumonectomy had been performed for residual lung adenocarcinoma 11 years before the presentation in question. The patient was elderly and had a poor pulmonary function, although her performance status and her cardiac function were good. Therefore, we decided to improve the safety of surgery with venovenous extracorporeal membrane oxygenation (VV-ECMO). Segmentectomy of S6 + S10a was performed under VV-ECMO support. The 30 months after surgery, the patient has had no complications but continues home oxygen therapy. Imaging has shown no evidence of recurrence.
INTRODUCTION
Several reports have described subsequent pulmonary surgery after pneumonectomy, some of which have indicated that subsequent wedge resection after pneumonectomy has a good prognosis. However, subsequent resection of the contralateral lung, including multiple wedge resection, segmentectomy and lobectomy, has not been associated with a good prognosis after pneumonectomy [1, 2]. Extended resection is associated with frequent complications; it is therefore important to improve the safety of these surgeries and to assess a low pulmonary function correctly in order to reduce postoperative complications. We herein report a case of uneventful subsequent segmentectomy of the left lung with venovenous extracorporeal membrane oxygenation (VV-ECMO) after right pneumonectomy.
CASE REPORT
An asymptomatic 82-year-old woman with no smoking history presented with a history of two surgeries of the right lung. She had undergone surgery for lung adenocarcinoma of the right upper lobe at another institution in 1990 and for residual right lung adenocarcinoma at our institution in 1996. Follow-up had been performed at another hospital since 2008. In September 2013, nodules were discovered in the patient’s left lower lobe on follow-up computed tomography (CT); she returned to our institution for further care. The nodules in the left lung had grown, and several new nodules had appeared. CT showed five nodules in the lower lobe of the left lung, all of which were located in segments 6 and 10a (Fig. 1). The size of the largest nodule was 2.1 cm. These nodules were suspected to be synchronous multiple lung cancer, because each had a ground-glass appearance. On respiratory function tests, the patient had a vital capacity (VC) of 840 ml, %VC of 45.7%, forced expiratory volume in 1 s (FEV1.0) of 610 ml and %FEV1.0 of 72.6%. However, the patient was able to climb stairs and did not have shortness of breath. In the assessment of the cardiac function, electrocardiogram showed regular sinus rhythm, and no ischemic changes. Echocardiogram showed an ejection fraction of 84%, no right heart strain and good wall motion.
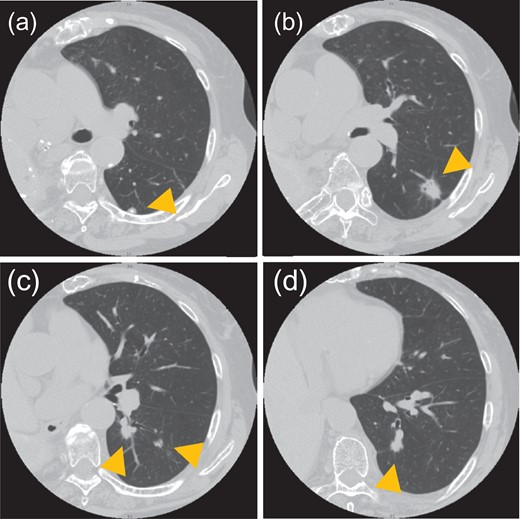
CT shows five nodules in the left lower lobe. Tumor sizes and locations: (a) 0.8 cm in S6a, (b) 2.1 cm in S6a, (c) 1.3 cm and 0.8 cm in S6c and (d) 1.1 cm in S10a. All nodules were located in S6 or S10a.
As a therapeutic strategy, surgical treatment was considered for three reasons. First, based on the CT characteristics, the tumors were suspected to be primary lung cancer rather than metastasis of previous right lung cancer. Second, it was difficult to perform radiation therapy because of the tumor location. Third, the patient’s performance status was adequate. With segmentectomy of S6 + S10a, the predicted postoperative VC and FEV1.0 were 510 and 366 ml, respectively. These values suggested that home oxygen therapy might be needed, and the patient accepted that.
Segmentectomy of S6 + S10a via thoracotomy was performed under VV-ECMO support. Blood was drained from the right femoral vein and returned to the right internal jugular vein. After disconnecting A6, V6 and B6, we severed A10a, V10a and B10a. An auto-suture instrument was used to divide the lung segments. The operation time was 206 min; blood loss was 245 ml. The time of VV-ECMO use was 258 min. A pathological examination revealed that all five tumors were adenocarcinoma with mixed subtypes, including 80–100% lepidic growth patterns. Each tumor was diagnosed as synchronous multiple lung cancer, pT1aN0M0 Stage IA.
When the patient started rehabilitation, she experienced dyspnea during activity. Therefore, the decision was made to use home oxygen. On postoperative Day 34, the patient was transferred to another hospital to continue further rehabilitation. She was discharged from the hospital and returned home on postoperative Day 48.
After 6 months of surgery, the patient’s cardiac contractile function was good, with an ejection fraction of 63.5% and mild pulmonary hypertension; the right ventricular systolic pressure was 43.5 mmHg. Although there was slight pressure overload, no right-sided heart enlargement or compression of the left ventricle were observed. The patient has continued home oxygen therapy. Imaging findings have shown no evidence of recurrence in the 43 months since the surgery.
DISCUSSION
Reports of contralateral lung surgery after pneumonectomy indicate that the average age at second surgery is 57.1 years, the average period between first and second surgeries is 69.5 months, and the average VC and FEV1.0 of patients are 2305 and 1699 ml, respectively [2–4] (Table 1). In the current case, the patient was in worse condition than those described in previous reports. Therefore, a careful evaluation and presurgical treatment were needed when making decisions about surgery. We discuss alternative therapeutic options with patients, including chemotherapy, radiation therapy and EGFR-TKI if patients have any mutations. However, these treatments require a diagnosis of lung cancer, and such a diagnosis requires performing a CT-guided biopsy or transbronchial lung biopsy. Furthermore, when we discuss performing these invasive studies for the diagnosis, patients typically express a desire to undergo surgery instead of these studies.
There have been no reports of the use of extracorporeal circulation in lung surgery after pneumonectomy [3]. In previous reports, bronchial blockers were used for respiratory support [2, 4, 5]. Although blockers are useful, they would not have collapsed the target segment of lung adequately in the current case. We therefore stopped ventilation in order to collapse the lung during surgery on VV-ECMO, so the operative view while stopping ventilation was much better than the view using blocker. VV-ECMO is a useful procedure for performing surgery safely. VV-ECMO has been used for patients with respiratory risk, including those undergoing tracheal dilation surgery and those with a low pulmonary function [6]. The indications for VV-ECMO use during surgery have not been clearly defined.
| Author . | Year . | Patient . | First operation site . | Histrogy . | Interval between operations (month) . | VC (ml) . | FEV1.0 (ml) . | Operative procedure . | Respiratory support . | Histrogy . | HOT . | Prognosis (month) . | Cause of death . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kayawake | 2015 | 72 males | Left | Sq | 37 | 1730 | 1360 | Open wedge | Meta | − | 14 death | Recurrence | |
| 66 males | Left | Mucinous BAC | 36 | 2760 | 2560 | Open wedge | Meta | + | 43 death | Pneumonia | |||
| 71 males | Left | MALT lymphoma | 240 | 2500 | 1530 | VATS S1seg | Ad primary | − | 39 death | Fulminant hepatitis | |||
| Kataoka | 2008 | 45 males | Right | Sq | 60 | 1950 | 1180 | Open wedge | Ad primary | − | 17 alive | ||
| Sakurai | 2003 | 30 females | Left | Adenoid cystic ca. | 53 | 2880 | 2490 | Open wedge | Blocker | Meta | − | 78 alive | |
| 41 males | Left | Ad | 9 | 2210 | 1660 | Open wedge x2 | Ad primary | − | 15 alive | ||||
| 64 males | Right | Ad | 32 | 2180 | 1680 | Open wedge | Ad primary | Temporarily | 63 death | Recurrence | |||
| 65 males | Left | Sq | 7 | 2350 | 1630 | VATS wedge | Blocker | Sq | − | 5 death | Respiratory dysfunction | ||
| Inoue | 1998 | 65 males | Left | Sq | 81 | 2930 | 1760 | Open wedge | Large primary | − | 42 alive | ||
| 64 males | Left | Large | 98 | 2410 | 1590 | Open wedge | Ad primary | − | 12 alive | ||||
| 35 females | Right | Ad | 24 | 2050 | 1660 | Open Upper seg | Meta | + | 17 alive | ||||
| Tajiri | 1998 | 67 males | Left | Ad | 157 | 1710 | 1290 | VATS wedge | Blocker + HFJV | Ad meta | − | 12 alive |
| Author . | Year . | Patient . | First operation site . | Histrogy . | Interval between operations (month) . | VC (ml) . | FEV1.0 (ml) . | Operative procedure . | Respiratory support . | Histrogy . | HOT . | Prognosis (month) . | Cause of death . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kayawake | 2015 | 72 males | Left | Sq | 37 | 1730 | 1360 | Open wedge | Meta | − | 14 death | Recurrence | |
| 66 males | Left | Mucinous BAC | 36 | 2760 | 2560 | Open wedge | Meta | + | 43 death | Pneumonia | |||
| 71 males | Left | MALT lymphoma | 240 | 2500 | 1530 | VATS S1seg | Ad primary | − | 39 death | Fulminant hepatitis | |||
| Kataoka | 2008 | 45 males | Right | Sq | 60 | 1950 | 1180 | Open wedge | Ad primary | − | 17 alive | ||
| Sakurai | 2003 | 30 females | Left | Adenoid cystic ca. | 53 | 2880 | 2490 | Open wedge | Blocker | Meta | − | 78 alive | |
| 41 males | Left | Ad | 9 | 2210 | 1660 | Open wedge x2 | Ad primary | − | 15 alive | ||||
| 64 males | Right | Ad | 32 | 2180 | 1680 | Open wedge | Ad primary | Temporarily | 63 death | Recurrence | |||
| 65 males | Left | Sq | 7 | 2350 | 1630 | VATS wedge | Blocker | Sq | − | 5 death | Respiratory dysfunction | ||
| Inoue | 1998 | 65 males | Left | Sq | 81 | 2930 | 1760 | Open wedge | Large primary | − | 42 alive | ||
| 64 males | Left | Large | 98 | 2410 | 1590 | Open wedge | Ad primary | − | 12 alive | ||||
| 35 females | Right | Ad | 24 | 2050 | 1660 | Open Upper seg | Meta | + | 17 alive | ||||
| Tajiri | 1998 | 67 males | Left | Ad | 157 | 1710 | 1290 | VATS wedge | Blocker + HFJV | Ad meta | − | 12 alive |
The 75% of initial surgeries were left pneumonectomy. Average age at subsequent pulmonary surgery was 57.1 years.
Sq, squamous cell carcinoma; Ad, adenocarcinoma; BAC, bronchioloalveolar adenocarcinoma; large, large cell carcinoma, VATS, video assisted thoracic surgery; HFJV, high frequency jet ventilation; meta, metastasis.
| Author . | Year . | Patient . | First operation site . | Histrogy . | Interval between operations (month) . | VC (ml) . | FEV1.0 (ml) . | Operative procedure . | Respiratory support . | Histrogy . | HOT . | Prognosis (month) . | Cause of death . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kayawake | 2015 | 72 males | Left | Sq | 37 | 1730 | 1360 | Open wedge | Meta | − | 14 death | Recurrence | |
| 66 males | Left | Mucinous BAC | 36 | 2760 | 2560 | Open wedge | Meta | + | 43 death | Pneumonia | |||
| 71 males | Left | MALT lymphoma | 240 | 2500 | 1530 | VATS S1seg | Ad primary | − | 39 death | Fulminant hepatitis | |||
| Kataoka | 2008 | 45 males | Right | Sq | 60 | 1950 | 1180 | Open wedge | Ad primary | − | 17 alive | ||
| Sakurai | 2003 | 30 females | Left | Adenoid cystic ca. | 53 | 2880 | 2490 | Open wedge | Blocker | Meta | − | 78 alive | |
| 41 males | Left | Ad | 9 | 2210 | 1660 | Open wedge x2 | Ad primary | − | 15 alive | ||||
| 64 males | Right | Ad | 32 | 2180 | 1680 | Open wedge | Ad primary | Temporarily | 63 death | Recurrence | |||
| 65 males | Left | Sq | 7 | 2350 | 1630 | VATS wedge | Blocker | Sq | − | 5 death | Respiratory dysfunction | ||
| Inoue | 1998 | 65 males | Left | Sq | 81 | 2930 | 1760 | Open wedge | Large primary | − | 42 alive | ||
| 64 males | Left | Large | 98 | 2410 | 1590 | Open wedge | Ad primary | − | 12 alive | ||||
| 35 females | Right | Ad | 24 | 2050 | 1660 | Open Upper seg | Meta | + | 17 alive | ||||
| Tajiri | 1998 | 67 males | Left | Ad | 157 | 1710 | 1290 | VATS wedge | Blocker + HFJV | Ad meta | − | 12 alive |
| Author . | Year . | Patient . | First operation site . | Histrogy . | Interval between operations (month) . | VC (ml) . | FEV1.0 (ml) . | Operative procedure . | Respiratory support . | Histrogy . | HOT . | Prognosis (month) . | Cause of death . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kayawake | 2015 | 72 males | Left | Sq | 37 | 1730 | 1360 | Open wedge | Meta | − | 14 death | Recurrence | |
| 66 males | Left | Mucinous BAC | 36 | 2760 | 2560 | Open wedge | Meta | + | 43 death | Pneumonia | |||
| 71 males | Left | MALT lymphoma | 240 | 2500 | 1530 | VATS S1seg | Ad primary | − | 39 death | Fulminant hepatitis | |||
| Kataoka | 2008 | 45 males | Right | Sq | 60 | 1950 | 1180 | Open wedge | Ad primary | − | 17 alive | ||
| Sakurai | 2003 | 30 females | Left | Adenoid cystic ca. | 53 | 2880 | 2490 | Open wedge | Blocker | Meta | − | 78 alive | |
| 41 males | Left | Ad | 9 | 2210 | 1660 | Open wedge x2 | Ad primary | − | 15 alive | ||||
| 64 males | Right | Ad | 32 | 2180 | 1680 | Open wedge | Ad primary | Temporarily | 63 death | Recurrence | |||
| 65 males | Left | Sq | 7 | 2350 | 1630 | VATS wedge | Blocker | Sq | − | 5 death | Respiratory dysfunction | ||
| Inoue | 1998 | 65 males | Left | Sq | 81 | 2930 | 1760 | Open wedge | Large primary | − | 42 alive | ||
| 64 males | Left | Large | 98 | 2410 | 1590 | Open wedge | Ad primary | − | 12 alive | ||||
| 35 females | Right | Ad | 24 | 2050 | 1660 | Open Upper seg | Meta | + | 17 alive | ||||
| Tajiri | 1998 | 67 males | Left | Ad | 157 | 1710 | 1290 | VATS wedge | Blocker + HFJV | Ad meta | − | 12 alive |
The 75% of initial surgeries were left pneumonectomy. Average age at subsequent pulmonary surgery was 57.1 years.
Sq, squamous cell carcinoma; Ad, adenocarcinoma; BAC, bronchioloalveolar adenocarcinoma; large, large cell carcinoma, VATS, video assisted thoracic surgery; HFJV, high frequency jet ventilation; meta, metastasis.
The incidence of complications with ECMO is 32–40%. Of these complications, 65–77% are bleeding and vascular injury accompanying cannulation. In contrast, vascular complications occur in 88% of patients receiving venoarterial ECMO [7], which is always used when long-duration ECMO is needed. Therefore, using ECMO only during surgery may reduce the risk of complications, except for those resulting from cannulation. Furthermore, venous cannulation is less invasive than arterial cannulation [8].
In conclusion, subsequent lung surgery after pneumonectomy requires an adequate pulmonary function. For patients in a poor condition, VV-ECMO is one option to improve the safety of surgery.
CONFLICT OF INTEREST STATEMENT
None declared.



