-
PDF
- Split View
-
Views
-
Cite
Cite
Enrico Coppola Bottazzi, Adele Noviello, Nicola Moles, Antonio Miro, Augusto Striano, Mario Baiamonte, Francesco Esposito, Francesco Crafa, Merkel cell carcinoma: extended lymphadenectomy and reconstruction with biosynthetic prosthesis, Journal of Surgical Case Reports, Volume 2018, Issue 5, May 2018, rjy098, https://doi.org/10.1093/jscr/rjy098
Close - Share Icon Share
Abstract
Merkel cell carcinomas (MCC) is an aggressive neuroendocrine carcinoma originating from the Merkel cell in the dermo-epidermal junction. Only 10% of MCC occur on the skin of the trunk.
We report a case of Merkel’s abdominal carcinomas treated with extensive inguinal lymphadenectomy and reconstruction of the abdominal wall and inguinal canal using prosthesis GORE® BIO-A®.
Immunohistochemical analysis by tumor-specific markers is crucial for diagnosis and permits differentiation from other tumors of the skin. MCC is an aggressive tumor with poor prognosis.
For primary tumors without indications of the presence of organ metastases complete surgical excision is the gold standard. Gore BIO-A is a biosynthetic prosthesis with manageable structure that allows it to be positioned and shaped according to needs, its strength provides for excellent support for the reconstruction of the inguinal canal wall.
INTRODUCTION
Freidrich Sigmund Merkel first described the Merkel cell in 1875. Merkel cell carcinoma (MCC) was originally described by Toker in 1972 as trabecular carcinoma of the skin [1].
MCC is an aggressive neuroendocrine carcinoma originating from the Merkel cell in the dermo-epidermal junction, which belongs to the amine precursor uptake and decarboxylation (APUD) system.
The median age at diagnosis is ~65 years. Incidence is considerably greater in whites than blacks and slightly greater in males than females [2].
The exact aetiology is unknown. Scientific evidence also shows a strong link between MCC and ultraviolet light exposure (UV). MCC is closely associated with squamous cell carcinoma (SCC), basal cell carcinoma (BCC) and Bowen’s disease (a first, superficial SCC variant), all of which are most frequently caused by exposure to UV rays. An increase in MCC incidence has also been observed in people with chronic immune suppression [3].
Feng et al. [4] scientists at the University of Pittsburgh discovered a ‘Merkel cell polyomavirus’ MCV o McPyV which may be a contributing factor to MCC pathogenesis. High levels of viral DNA and clonal integration of the virus in MCC tumors have also been reported along with expression of certain viral antigens in MCC cells and the presence of antiviral antibodies.
Approximately 50% of MCCs occur on the head and neck (46% of these in the periorbital region), 35% on the extremities and 10% on the skin of the trunk [5].
We report a case of Merkel’s abdominal carcinomas treated with extensive inguinal lymphadenectomy and reconstruction of the abdominal wall and inguinal canal using biosynthetic prosthesis.
CASE REPORT
A 58-year-old man, with a recent history of polyglobuline treated with phlebotomies, first degree obesity and multinodal goiter. After the appearance of a subcutaneous swelling of the left abdominal wall, extended by the umbilical line for ~6 cm, of solid consistency and fixed to the fascia, ultrasound imaging and a biopsy of the removal of the abdomen were performed.
Histological examination described the presence of a malignant neoplasia of medium-sized cells, with nucleated chromatin, dispersed in nests and cords; with high mitotic activity and apoptotic index. Immunohistochemical study was positive for Citokeratina (CK) 20, CKpan, CD56, chromogranin, sinaptofisine, CD44, neurofilaments (dot-like, partial) and Pax5 (weak and partial) and negative for Vimentina, S100, CK7, CD117, CD99, TdT and thyroid transcription factor 1 (TTF1). Diagnosis was of Merkel’s skin neuroendocrine carcinoma.
A positron emission tomography (PET) with CT-scan reconstruction showed hyper metabolic at the right breast nodule of 1.8 cm (SUV 3.7), at the left abdominal mass of 6 cm (SUV 9.37) and at superficial (SV 9.6) and deep (SUV 8.5) inguinal lymph node (Fig. 1).
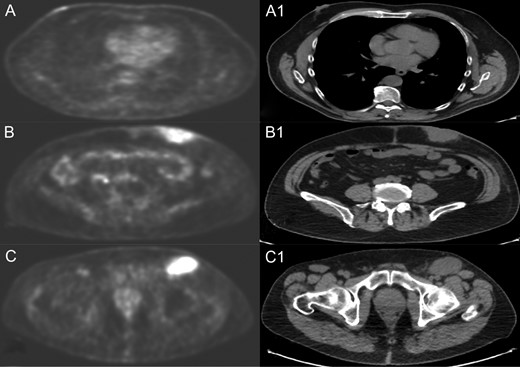
PET and CT scan of right breast nodule (A and A1), left abdominal mass (B and B1) and left inguinal lymph node (C and C1).
Then a right mastectomy with third level axillary lymphadenectomy, for sentinel lymph node positivity; removal of the left abdominal mass with resection of the band of external oblique muscle and of the infiltrated anterior inguinal wall; lymphadenectomy of the external and common left iliac artery; deep and superficial left inguinal lymphadenectomy (Fig. 2) were performed. Reconstruction of the abdominal band and of the inguinal canal wall was obtained using a GORE® BIO-A® prosthesis (Fig. 3).
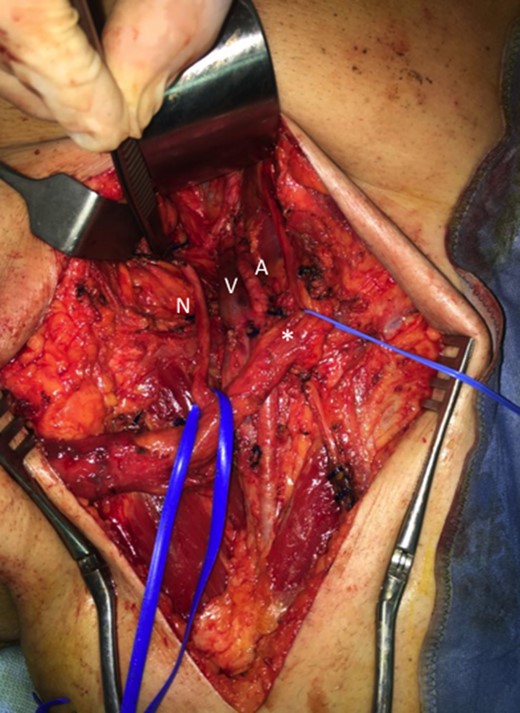
Inguinal region after linfoadenectomy (N, nerve; V, Vein; A, Artery; *spermatic cord).
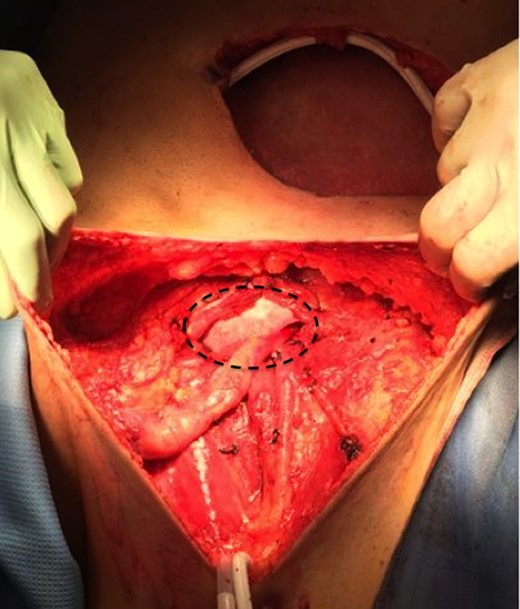
GORE® BIO-A® prosthesis used for reconstruction of the abdominal wall and inguinal canal.
Final histological examination confirmed an abdominal MCC with inguinal and axillary massive lymph node metastasis T3 N1b M1a, Stage IV. The right breast nodule was a melanocytic nerve.
According to the oncologists and radiotherapists, the patient was not subjected to any therapy; 10-month follow-up there is no evidence of recurrence. We did not observe inguino-crural hernias after 10 months.
DISCUSSION
Immunohistochemical analysis by tumor-specific markers is crucial for diagnosis and permits differentiation from other tumors of the skin. MCC is positive for Cytokeratin (CK) 20, neuro-filament and neuron specific enolase (NSE); negative to CK 7, Thyroid Transcription Factor (TTF) 1, S100 Protein, leukocyte commion agent (LCA) whereas melanoma is only positive for S100 protein and Lymphoma only for LCA [6].
Fine needle aspiration cytology (FNAC) enables an early noninvasive diagnosis of this aggressive tumor to facilitate early planning for surgery [7].
Loco regional lymph nodes represent the most frequent spread of MCC, highly predictive of negative prognosis tumor. Sentinel lymph nodes (SLN) should only be used in patients without clinical evidence of lymph node involvement, enabling a correct staging of the disease and imposing a more invasive surgical step only in patients who show lymph node metastases. The sentinel lymph node appears to be a specific and sensitive prognostic index in the MCC [8].
Surgery is the mainstay of treatment for MCC. Wide local excision with 1–2 cm margins to the investing fascia layer remains the standard surgical technique. Radiotherapy is an inferior option for cancer control, since the complete response is only 75% [9].
Gore Bio-A tissue reinforcement is a 3D web of completely absorbable synthetic polymers replaced by soft tissue within 6 months; It is a mix of glycolic acid and trimethylene carbonate and its function consists of rather than producing a mechanical barrier a stimulation of collagens deposition and ingrowths of new connective soft tissue occurs (Fig. 4) [10].

Gore Bio-A tissue reinforcement and 3D aspect (Images provided courtesy of W. L. Gore & Associates.).
Gore BIO-A has a manageable structure that allows it to be positioned and shaped according to needs, its strength provides for excellent support for the reconstruction of the inguinal canal wall (Fig. 3) as in our case, where the front wall of the inguinal canal and the external inguinal ring have been removed for neoplastic infiltration.
CONCLUSION
MCC is an aggressive tumor with poor prognosis. For primary tumors without indications of the presence of organ metastases complete surgical excision is the gold standard. When micrometastases are found in the sentinel lymph node, this should be followed by complete lymphadenectomy. MCCs are usually radiosensitive; Retrospective analyses show that the high local recurrence rate after R0 surgery of the primary tumor alone can be reduced significantly by combined loco regional adjuvant radiation therapy. In advanced cases with radiological evidence of lymph node diffusion, as in our case, surgical resection and extensive loco regional lymphadenectomy are indicated.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- carcinoma
- epidermis
- merkel cell carcinoma
- carcinoma, neuroendocrine
- inguinal canal
- lymph node excision
- merkel cells
- neoplasm metastasis
- reconstructive surgical procedures
- abdomen
- diagnosis
- neoplasms
- skin
- lymph node dissection
- abdominal wall
- inguinal lymphadenectomy
- excision
- gold standard
- trunk structure
- prostheses



