-
PDF
- Split View
-
Views
-
Cite
Cite
Sinan Khadhouri, Dupinderjit Singh Rye, Manish Powari, Ian R Daniels, John S McGrath, A case report of squamous cell carcinoma in a suprapubic urinary catheter tract: surgical excision and simultaneous colostomy formation, Journal of Surgical Case Reports, Volume 2018, Issue 2, February 2018, rjy030, https://doi.org/10.1093/jscr/rjy030
Close - Share Icon Share
Abstract
Squamous cell carcinoma (SCC) arising from a suprapubic cystostomy tract is a rare complication of long-term suprapubic catheterization (SPC). A 53-year-old man with paraplegia secondary to spina bifida presented with a painful granulomatous lesion around his SPC site that was being treated with silver nitrate cauterization in the community. Consequently, he developed a sacral pressure sore due to reduced mobility from the pain. He also had increasing difficulties with defaecation secondary to his spina bifida. His sacral pressure sore was secondary to a cryptoglandular fistula with coccygeal osteomylelitis. Post-operative pathology revealed infiltrative SCC involving full thickness of the specimen from skin to the bladder wall with clear surgical margins. We describe the first case requiring a simultaneous suprapubic tract SCC excision and colostomy formation. We recommend early investigation of lesions arising from a long-term suprapubic tract especially in patients with spinal cord injuries or congenital defects.
INTRODUCTION
Squamous cell carcinoma (SCC) of the bladder represents a small proportion of bladder cancers in the West, the incidence varying from 2 to 5% [1]. However, the incidence increases with concomitant spinal cord injuries particularly in those with long-term catheters [1, 2].
SCC arising from the site of a suprapubic catheter (SPC) tract, with or without involvement of the bladder urothelium, is very poorly represented in literature with minimal studies to date [3–9].
CASE REPORT
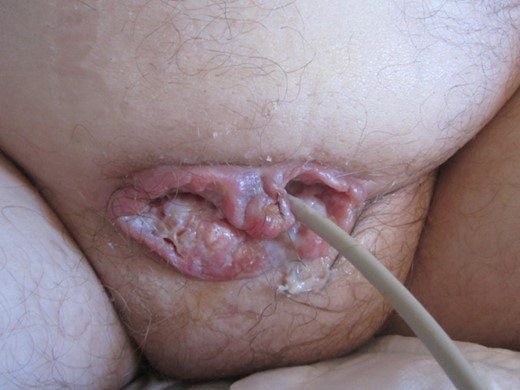
A 53-year-old, wheelchair-bound man with a long-term SPC (20 years) and paraplegia secondary to spina bifida presented to his GP with painful catheter changes, recurrent urinary tract infections (Pseudomonas) and a granulomatous lesion around his SPC site. He had no other co-morbidities and had sensation only to the level of L1/2. The lesion was treated with silver nitrate cauterization in the community but it continued to grow over the ensuing 2–3 months period. He was referred urgently to the urology outpatient clinic for assessment. Examination revealed a granulomatous growth around his suprapubic site, protruding 2–3 cm (Fig. 1). A biopsy was taken, which revealed an infiltrating, moderately differentiated SCC. A staging CT scan was negative for metastatic disease. A flexible cystoscopy excluded any obvious bladder mucosal involvement. The findings were discussed in the uro-oncology multi-disciplinary team (MDT) meeting and an elective excision was planned.
However, as a result of the pain that the patient was experiencing, regular position changes were not maintained, leading to the development of a Grade 4 (full thickness tissue loss with exposed bone) sacral pressure sore. This ultimately resulted in an emergency surgical admission ahead of his planned operation date as he had developed signs of sepsis. On examination, the sacral sore measured 3 cm in depth and 15 cm wide, with visible bone and surrounding cellulitis. Clinically, there were no signs of necrotizing fasciitis. The patient was started on intravenous antibiotics and his pressure sore managed by the tissue viability nursing team. A repeat CT scan was performed and this demonstrated air within the left ischial rectal fossa with evidence of osteomyelitis in the coccygeal bones (Fig. 2). A joint review was undertaken by the colorectal and urology team as an inpatient. Due to the proximity of the anus to the sacral pressure sore and the patient’s chronic problem with constipation (requiring manual evacuation), it was decided after discussion with the patient that an end colostomy should be performed alongside the excision of SCC.
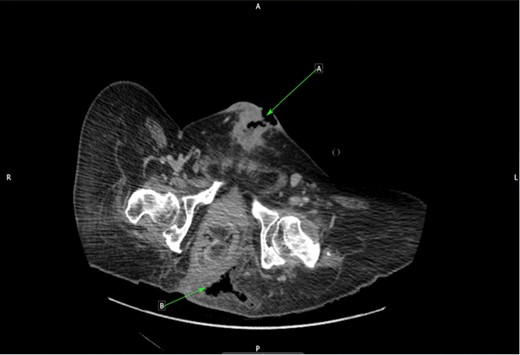
CT cross-sectional plane of pelvis. (a) Infiltrating SCC along suprapubic tract. (b) Air in left ischiorectal space from sacral sore.
The patient’s condition improved following antibiotic therapy and wound care. Seven days post admission, he underwent an excision of SCC, abdominoplasty, formation of an end colostomy and debridement of sacral sores.
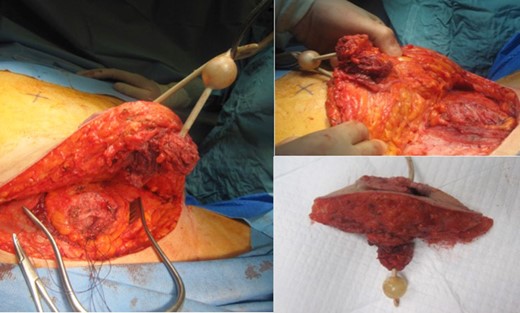
The SCC was excised en-bloc with a partial cystectomy through a modified elliptical incision. Included in the excision was skin, fat, rectus sheath, rectus muscle and bladder cuff (Fig. 3). The incision was then extended superiorly as a Fleur-de-Lys to allow for a laparotomy and colostomy formation. The descending colon was found to be obstructed with hard stool that was evacuated through the stoma. A Fleur-de-Lys abdominoplasty was necessary to ensure appropriate skin closure, and the umbilicus was re-sited. A SPC was re-sited superior–lateral to the midpoint of the Fleur-de-Lys incision, with the bladder and abdomen closed in layers (Fig. 4). A percutaneous drain was left superficial to the anterior rectus sheath. Debridement of the sacral wound and coccygectomy were then performed, having identified a cryptoglandular fistula as the source.
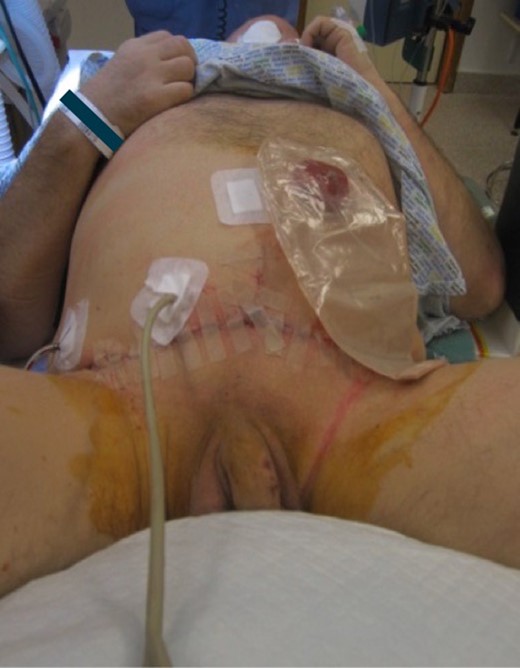
Post-operative image showing Fleur-de-Lys incision, SPC re-siting and colostomy.
The patient made a good post-operative recovery. His sacral wound was managed with negative pressure dressings and a seton suture. Pathologically, gross examination revealed an ulcerated tumour involving skin, which extended through the subcutaneous fat and appeared to communicate with the bladder cuff. Histology confirmed moderately differentiated SCC throughout the tract involving the bladder wall, with clear surgical margins (Fig. 5).
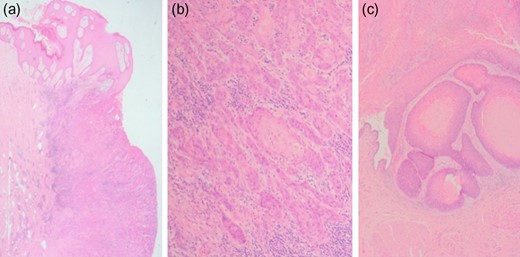
Histopathological images of SCC. Microscopy showed widely infiltrative squamous cell carcinoma involving full thickness of the specimen from skin to the bladder wall. No lymphovascular invasion was seen. The margins were clear although the deep detrusor muscle margin was clear by only 1 mm. (a) Photomicrograph showing abdominal wall skin with carcinoma (H&E ×1.25), (b) Photomicrograph showing infiltrating islands of squamous carcinoma in the subcutaneous tissue (H&E ×10), (c) Photomicrograph showing squamous carcinoma involving the bladder (H&E ×4).
The patient was reviewed 8 weeks later in clinic and was recovering well with no complications. His case was discussed at the MDT meeting where cystoscopic and cross-sectional surveillance was advised. At 8 months follow-up, the patient had no evidence of recurrence.
DISCUSSION
We discuss the first case report of an excision of SPC tract SCC and simultaneous colostomy formation. This required careful surgical planning and consideration had to be made to minimize the midline laparotomy incision as the patient relied heavily on his abdominal wall muscles when transferring himself from and to the wheelchair. A Fleur-de-Lys incision was thought to be the optimal approach to primarily excise the tumour through a lower elliptical incision, then allow for a colostomy by extending a midline incision only as was necessary to mobilize the colon and bring out a colostomy, with the abdominoplasty allowing formation of the stoma on a flat surface.
There are few case reports describing SCC arising from the suprapubic tract [3–9], most of them in patients with spinal cord injuries, with a long-term catheter in situ (ranging from 5 to 37 years). Bladder involvement amongst these is mixed. Our case showed a grossly normal bladder on cystoscopy but there was evidence of SCC in the bladder on histology. This demonstrates that a normal cystoscopy cannot rule out bladder involvement.
Treatment of these cases varied in the literature according to the patient’s co-morbidities and staging of the tumour. Most cases favoured surgical excision for locally advanced disease, or radiotherapy if the patient is not fit for surgery and/or has metastatic disease. Outcome of treatment was varied. The mortality rate seems to be high in general due to recurrence or complications of treatment, and usually occurred within a year following treatment [4, 5, 9].
Follow-up is therefore necessary, although the rarity of this disease means there are no set recommendations for frequency and type of surveillance, or indeed prognostic factors. The authors propose that this is decided in a local MDT setting.
The authors recommend early investigation of lesions arising from a suprapubic cystostomy tract, especially in long-term SPC over 5 years in patients with spinal cord injury or neurocongenital defects, to assist early detection and treatment of SCC.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- congenital abnormality
- squamous cell carcinoma
- defecation
- pathologic fistula
- cautery
- decubitus ulcer
- granuloma
- pain
- paraplegia
- silver nitrate
- spinal cord injuries
- spinal dysraphism
- colostomy procedure
- pathology
- skin
- cystostomy, suprapubic
- urinary catheters
- bladder wall
- surgical margins
- excision
- community
- mobility
- statistical process control



