-
PDF
- Split View
-
Views
-
Cite
Cite
Tashinga Musonza, Christy Y Chai, Non-operative management of iatrogenic colonic perforation after percutaneous cholecystotomy, Journal of Surgical Case Reports, Volume 2018, Issue 12, December 2018, rjy338, https://doi.org/10.1093/jscr/rjy338
Close - Share Icon Share
Abstract
The management of iatrogenic colonic perforation encountered during percutaneous cholecystotomy tube placement is not well reported. It is unclear as to whether an operative versus a conservative approach is ideal for this complication. We therefore present our case report to spur a discussion on patient selection, interval follow-up and call for future studies regarding this uncommon complication.
INTRODUCTION
Colonic perforation due to the placement of percutaneous cholecystotomy tubes is sparsely reported. As such there is no management guidelines or adopted algorithms. This poses a unique challenge to the consulted surgeon especially in a patient without overt peritonitis. Our case report underscores the feasibility of a non-operative approach for this complication. However, there are no defined parameters for optimum patient selection.
CASE REPORT
An 83-year-old male with a significant cardiac history and an indwelling biliary stent for chronic choledocholithiasis presented with acute on chronic abdominal pain 3 months after biliary stent exchange at our institution.
He had undergone coronary artery bypass graft (CABG) (1991, 1999) and percutaneous coronary intervention (PCI) in 2010. Most recently, he had suffered a non-ST elevated myocardial infarction (NSTEMI) due to in-stent-restenosis in late 2017. This was in the context of held clopidogrel for an anticipated endoscopic retrograde cholangiopancreatography (ERCP) for choledocholithiasis.
At the time of presentation, he was now status post (s/p) PCI with a bare metal stent SVG-RCA and was on apixaban for atrial fibrillation. His ejection fraction (EF) was 35–40%. He also had insulin dependent diabetes mellitus, stage III chronic kidney disease (CKD III), myasthenia gravis and a chronic choledocholith.
Upon evaluation, he had right upper quadrant pain, nausea, non-bloody non-bilious emesis and low-grade fevers (100.4 F). His laboratory studies revealed leukocytosis (WBC 23 000 ul/ml), troponemia (T 1.05), a normal lipase and liver function studies.
A computed tomography (CT) with intravenous contrast done in the emergency room had shown cholelithiasis without signs of cholecystitis, a known 7 mm stone in the proximal common bile duct (CBD), absence of intra or extra-hepatic dilation and peripancreatic fat stranding. His ultrasound showed gallbladder sludge, cholelithiasis, trace pericholecystic fluid, normal wall thickness and a known common bile duct stone. He had positive sonographic Murphy’s sign. A hepatobiliary iminodiacetic acid (HIDA) scan was done and this was consistent with cholecystitis (Fig. 1).
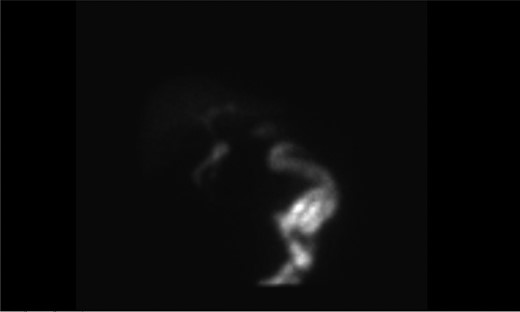
Our surgical team was consulted for the management of presumed cholecystitis. He had positive Murphy’s sign. His findings were consistent with acute cholecystitis and gallstone pancreatitis.
In the context of his active NSTEMI, prior NSTEMI upon holding antiplatelet therapy, heart failure with reduced ejection fraction (HFrEF) and a history of multiple PCIs we deemed him high risk for perioperative cardiac events. We therefore recommended a percutaneous cholecystotomy tube, broad spectrum antibiotics and nil per os (NPO).
Placement of the cholecystostomy tube was complicated by a through and through transverse colon perforation visualized upon fluoroscopic confirmation of cholecystotomy (Fig. 2). An 8 Fr catheter was reported to have been used. The interventionalist immediately withdrew the catheter from the colon and subsequently placed it in the gallbladder (Fig. 3). We were further consulted for the management of his colonic perforation.
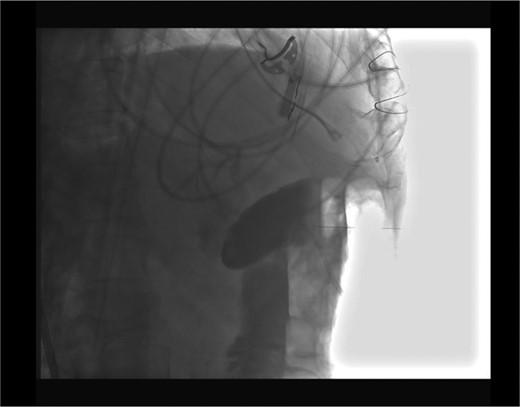
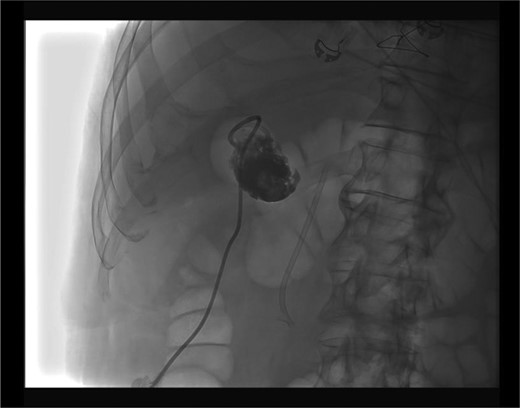
Upon examination, his vital signs were reassuring: no tachycardia, no fevers, no hypotension or tachypnea. His abdominal exam was unchanged and without generalized peritonitis. A decision for non-operative management with serial abdominal exams, antibiotics (piperacillin-tazobactam), intravenous fluids and NPO was chosen. Overnight, his abdominal exam was unchanged. The next day his abdominal exam was in fact better. A CT with rectal and oral contrast was done. This revealed no contrast extravasation.
The patient’s diet was subsequently advanced as his abdominal pain improved. He was discharged home in good condition.
DISCUSSION
Percutaneous cholecystotomy tube placement is generally indicated for the treatment of acute cholecystitis in high-risk patients with contraindications to general anesthesia [1]. The incidence and management of colonic perforation due to placement of cholecystotomy tubes are not well reported. Common complications such as bleeding, catheter dislodgment or blockage or failure to resolve cholecystitis have been reported to range from 10% to 15% [2].
The paucity of data addressing the incidence and management of patients after colonic perforation poses a challenge to the consultant. The surgeon is faced with a conundrum: operating on a very high-risk patient versus closely observing this patient with an understanding of the likelihood of having to operate if the patient deteriorates.
Our patient had active NSTEMI and apart from that he was high risk based on the revised cardiac index. He also had a history of NSTEMI upon interruption of antiplatelet therapy. An in-depth discussion had been held with the family and the consulting service regarding his medical status and surgical risk profile. All were in concordance with a non-operative plan.
Drawing from this experience, we think a non-operative approach may be feasible in managing iatrogenic colonic perforation due to percutaneous cholecystotomy tube placement. However, these select patients should be monitored closely for worsening clinical status: fevers, tachycardia, hypotension, worsening abdominal pain and peritonitis.
We do not necessarily think that an enteric or intravenous contrast study should be routinely done to document the absence or presence of extravasation if the patient is clinically doing well.
The fact that the caliber of cholecystostomy tubes is usually 8 Fr or smaller probably decreases the likelihood of frank abdominal contamination thus increasing the probability of successful non-operative management.
There is an extensive literature on the management of colonic perforation encountered during colonoscopy. Endoscopic maneuvers utilizing clips or endoloops have been reported [3, 4]. A large perforation or the development of leukocytosis, fever or worsening abdominal pain in patients managed with clips after colonic perforation during colonoscopy was predictive of the need for surgical intervention [5]. It is possible that a similar algorithm can be extrapolated for colotomy secondary to percutaneous cholecystotomy tube placement.
The utilization of percutaneous cholecystotomy tubes will probably increase with the ageing population and further retrospective and prospective studies may shed light in the ideal management of iatrogenic colonic perforation encountered during percutaneous cholecystotomy. In fact, our institution predominantly serves veterans whom often are elderly and medically high risk. An understanding of the management of colonic perforation in this population is of utmost importance to the surgeon’s armamentarium. Further retrospective and prospective studies may shed light on optimal patient selection for non-operative management.
CONFLICT OF INTEREST STATEMENT
None declared.



