-
PDF
- Split View
-
Views
-
Cite
Cite
Bent Are Hansen, Svein Amundsen, Håkon Reikvam, Øystein Wendelbo, Gustav Pedersen, Non-curative surgery for aortoenteric fistula, Journal of Surgical Case Reports, Volume 2017, Issue 8, August 2017, rjx153, https://doi.org/10.1093/jscr/rjx153
Close - Share Icon Share
Abstract
Graft infection with secondary aortic fistula is a rare complication following implantation of aortic prostheses, frequently occurring after emergency procedures and reoperations. The condition is associated with considerable morbidity and mortality. Curative treatment consists of explantation of the infected graft followed by fistula revision and implantation of a new graft in combination with antimicrobial therapy. Non-curative treatment with aortic stentgraft and long-term substitution treatment with antibiotics may be an option in cases where graft explantation is deemed too risky. We present an elderly patient with aortoenteric fistula following surgery for ruptured abdominal aortic aneurysm. Implantation of an aortic stentgraft and fistula revision was performed but the original aortic prosthesis was not explanted. The aortoenteric fistula recurred twice, but the patient has survived more than 12 years following non-curative surgery with good quality of life.
INTRODUCTION
Aortic graft infection with secondary aortoenteric fistula is a rare complication to aortic surgery, associated with increased morbidity and mortality [1, 2]. Early identification of the septic patient, appropriate diagnostic approach, source control, targeted antibiotic treatment and individualized surgery are key elements to successful treatment. This case report presents an unusual course following non-curative surgical treatment for aortoenteric fistula.
CASE DESCRIPTION
A 75-year-old woman with a history of cholecystectomy and surgery for breast cancer was admitted in 2003 with a 10 cm large ruptured infrarenal abdominal aortic aneurism (AAA). Immediate surgery with implantation of a polyester tube graft was performed. Postoperatively, sepsis due to Staphylococcus aureus occurred. The source was not identified, and Penicillin and Ciprofloxacin were administered intravenously for 3 weeks until discharge.
Three months postoperatively, she was admitted with sepsis and pain in her right foot. Computer tomography (CT) confirmed a plantar abscess which was treated surgically. Intravenous Ciprofloxacin and Cloxacillin were administered for 10 days followed by oral antibiotics for 1 week. Supplementary CT angiography (CTA) was performed, revealing a 4.2 cm large pseudoaneurysm at the cranial graft anastomosis. Microbiological cultures grew negative. The patient responded following 3 weeks of intravenous Cloxacillin. The pseudoaneurysm was followed with ultrasound every 6 months until a diameter of 5.2 cm in December 2004. To exclude the pseudoaneurysm, a stentgraft (Endurant®, Medtronic) was implanted in March 2005. Early onset postoperative sepsis complicated the course, and CTA revealed abscess formation in the pseudoaneurysm. CT-guided puncture was performed (Fig. 1). Cultures grew Streptococcus milleri, Eikenella corrodens and Bacterioides sp. Meropenem was administered with clinical response. Four weeks after stentgraft implantation, open revision of infected and necrotic tissue was performed. The graft implanted in 2003 and the stentgraft were partially exposed in a range from 2 to 5 cm distal from the renal arteries. A fistula from the last part of duodenum to the aneurysm sac was closed surgically. A decision was made not to explant the grafts due to impaired general condition and high age, and that graft excision would include suprarenal aortic clamping. Gentamycin (Gentacoll®) sponge was applied locally on the grafts and coverage with omentum was performed. Two weeks postoperatively, the patient was discharged with Ciprofloxacin and Clindamycin for 3 months. In June 2005, CTA revealed no sign of infection and antibiotics were continued for 6 months. In 2007, she presented with fever, and blood cultures grew Actinomyces. A decision was made to treat with antibiotics indefinitely. During the period 2008–13, the patient was admitted at several occasions with sepsis, successfully treated.
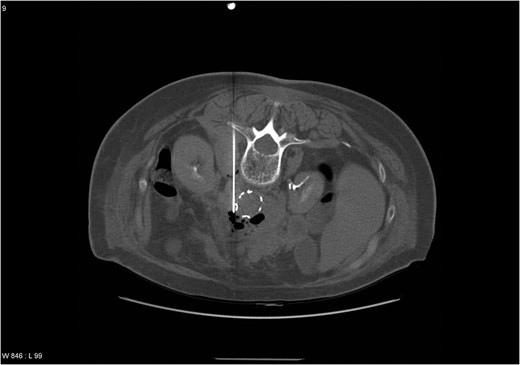
CT scan showing an abscess formation around the aortic stentgraft and a needle in the abscess for drainage. Note the presence of air bubbles in the abscess.
In December 2013, she was admitted with melena. Gastroscopy, CTA and sigmoidoscopy did not identify the bleeding source. The hemorrhage stopped spontaneously. Readmission occurred 2 weeks later due to hypovolemic shock. Endoscopic ultrasound revealed an aortoenteric fistula with a 3 × 2 cm2 opening with exposure of aortic graft material. Open surgery was considered too risky and a new stentgraft (Endurant®, Medtronic) was implanted in January 2014. For the next 2 years there were several admissions due to sepsis. In 2016, the patient was admitted due to hematemesis. Endoscopy visualized aortic graft material penetrating the duodenal wall (Fig. 2). The patient was stabilized and was discharged to her home.
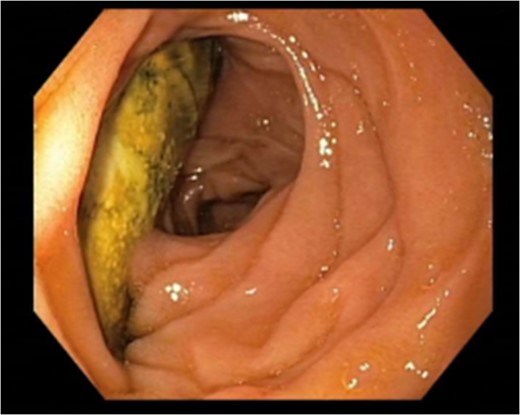
Endoscopy performed November 2016 revealing breakthrough of aortic graft material into the last part of the duodenum.
DISCUSSION
Aortic graft infection has an incidence of 0.5–6% with secondary aortoenteric fistula occurring in 20–45 % [2]. Risk factors include postoperative bacteremia, emergency surgery high-risk patient and redo surgery [3], present in this case except the latter. Heterogenecity of the patient population, different surgical methods and multitude of microbes involved make it difficult to compare studies [2]. However, operative mortality of 20–50% and survival rate of 50% at 24 months is reported [2, 4]. Aortoenteric fistula should be suspected if Gram-negative bacteria are present [2, 4]. Several different microorganisms were isolated in our patient, all commonly present in the digestive tract. Antibiotics should always be administered intravenously in an empiric fashion followed by targeted therapy with bactericidal activity to ensure high in vivo concentration, covering most common occurring microbes. Subsequently, treatment should be adjusted based on susceptibility testing of the recovered strains [3].
Aortic grafts are most susceptible to colonization of microbes early in the postoperative period [3]. This patient presented with bacteremia a few days after primary surgery for ruptured AAA. Relevant antibiotics were given but were discontinued. Shortly after discontinuation, she had a new flare which was treated with short-term antibiotics. These symptoms may have been linked to graft infection, although this was not verified initially. Recommended curative treatment strategy for chronic graft infection is mainly surgical as well as adequate antibiotic treatment [2]. Antibiotics may have suppressed but not eradicated the patient's infection. Life-long appropriate antibiotic therapy for any patient receiving a graft in an infected field is recommended [4].
Regarding curative surgery, there are two main methods. (i) In situ graft replacement using an antibiotic-soaked prosthesis, a silver prosthesis or a lower extremity vein graft restores circulation of the lower body and avoids leaving a closed aortic stump which carries a risk of blowout. (ii) Implantation of an axillofemoral bypass to maintain circulation of the lower limbs followed by aortic graft excision, leaving a closed infrarenal aortic stump. The second method has limited long-term patency but avoids lower limb ischemia during surgery, thereby decreasing operative risk. Our patient had significant risk factors at the time of diagnosis and it was therefore decided to refrain from graft explantation. Stentgrafts can reduce immediate risk, acting as bridging therapy in inaccessible areas, active bleeding and high-risk patients, as in this case. Subsequently, the aortoenteric fistula recurred and was treated with another stentgraft in 2014. It recurred once again in 2016 (Fig. 2) to be left to conservative treatment only.
Surprisingly, the patient has survived more than 14 years after surgery for ruptured AAA and 12 years after non-curative surgery for aortoenteric fistula.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
Author notes
Both authors contributed equally.



