-
PDF
- Split View
-
Views
-
Cite
Cite
Enio Campos Amico, José Roberto Alves, Samir Assi João, Joafran Alexandre Costa de Medeiros, Rogério Lacerda Sousa, Venous occlusion test applied to the tributaries of the superior mesenteric veins of the pancreas head infiltrated by tumor, Journal of Surgical Case Reports, Volume 2017, Issue 6, June 2017, rjx109, https://doi.org/10.1093/jscr/rjx109
Close - Share Icon Share
Abstract
A 64-year-old white woman presented with cholestatic jaundice, weight loss and a solid lesion in the pancreas head. At multislice computed tomography, a superior mesenteric vein (SMV) and one of it tributaries showed signs of tumor infiltration. At surgery, a venous occlusion test applied to the infiltrated tributary of the SMV showed immediate venous congestion in two-thirds of the distal small intestine. No reconstruction attempt was made due to the small size of the vessel. A biliodigestive anastomosis and lymph node biopsy was performed. The herein assessed case report suggests that the ileal tributary occlusion test applied to patients presenting pancreatic adenocarcinoma, with invasion of the tributaries of the SMV, may be effective in contraindicating resection procedures.
INTRODUCTION
The venous resection associated with pancreatoduodenectomy (PD) is widely performed in patients presenting pancreatic adenocarcinoma (PA) and mesenteric-portal axis (MPA) invasion. Previous studies have demonstrated that this procedure provides 5-year survival to some patients [1–4]. Resections proximal to the portal vein (PV) and to the superior mesenteric vein (SMV) are technically simple surgical procedures; however, great difficulty is expected when the tumor invades the SMV tributaries [4]. In addition to their small diameter, these tributaries tend to have thinner walls that makes difficult to reconstruct them. Thus, the management varies from simple ligation of the affected venous tributary to, whenever possible, its reconstruction. There are cases in which the resection is contraindicated. Our aim is to present a vascular test in order to help the decision-making about the best intraoperative approach to management to this cases.
CASE REPORT
A 64-year-old white woman presenting cholestatic jaundice and weight loss. A solid lesion (3.5 × 2.2 cm2) in contact to the SMV posterior wall in the inferior border of the pancreas head was identified through multislice computed tomography. The 3D CT vascular reconstruction showed that SMV was formed by two ileal trunks, and that was a tumor in contact to one of them (Fig. 1). There was no tumor in contact with the superior mesentery artery. An upper digestive endoscopy and serum Ca 19-9 (2740 U/ml) were performed. The patient was submitted to preoperative enteral nutrition for 22 days. Tumor infiltration into the pancreatic head and into one of the SMV ileal tributaries was confirmed during surgery. The vascular clamp occlusion test showed immediate venous congestion in two-thirds of the distal small intestine (Fig. 2). No reconstruction attempt was performed, as well as the resection of the small intestine segment, due to the small size of the vessel and the reduced amount of viable residual intestine. A pancreatic-duodenal lymph node biopsy (frozen section examination: adenocarcinoma) and a biliodigestive anastomosis were carried out. In the postoperative period, the patient presented persistent vomiting, and a gastric bypass was performed. The patient was discharged 8 days later and referred for chemotherapy.
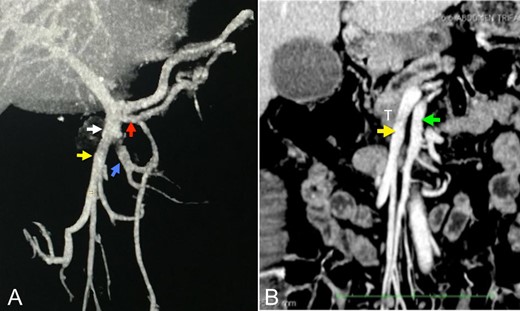
(A and B) Angiotomography showing the venous reconstructions of the portal-mesenteric axis and superior mesenteric artery in the coronal plane. White arrow: ileal trunk of the superior mesenteric vein. Red arrow: first jejunal branch (5.5 mm). Yellow arrow: venous tributary of the ileal venous ‘trunk’ involved by the tumor (9.6 mm). Blue arrow: venous tributary of the ileal trunk (9.3 mm). White arrow: ileal venous ‘trunk’. Green arrow: superior mesenteric artery (8 mm). T: tumor. It is important observing that, although the ileal venous ‘trunk’ involved by the tumor (yellow arrow) has the same diameter of the other ileal venous ‘trunks’ (blue arrow), a larger number of small tributary veins is observed in the first trunk.
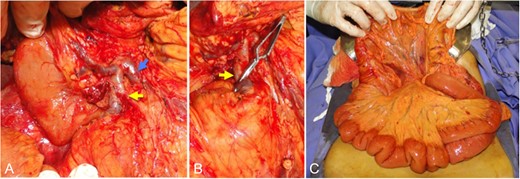
Intraoperative pictures. (A) Aspect after pancreas tumor dissection and the tributaries of the superior mesenteric vein. (B) Application of the vascular clamp in venous tributary involved by the tumor. (C) Aspect of the intravenous congestion in the small intestine mesentery after the clamping procedure (venous occlusion test). Yellow arrow: venous tributary of the ileal trunk involved by the tumor. Blue arrow: venous tributary of the ileal venous ‘trunk’ free from the tumor. T: tumor.
DISCUSSION
The technical aspects related to PD in patients with pancreatic neoplasms and infiltration of the SMV tributaries have been poorly documented in the literature and there is a lack of consensus about the treatment options to the aforementioned medical condition [5–7]. Katz et al. [5], for instance, indicates the MD Anderson group approach when only one of the two venous trunks forming the SMV are compromised. When such condition is found, he suggests a ligation without reconstruction, as long as the other tributary is of good caliber. Nonetheless the National Comprehensive Cancer Network Guidelines recommendation is quite pessimistic and resection is not advised in this scenario [8].
Two aspects should be considered: (i) although there are some well-known studies on the anatomy of the SMV venous tributaries [9–11], there is lack of studies correlating such veins to the small intestine loop drained by them. (ii) Despite Katz et al.'s suggestion [5], there is no guarantee that the intestine venous drainage would be done through collaterals after the ligation of some first-order tributary. It is very reckless to say that this will indeed occur since the consequences of venous thrombosis of the small bowel in the postoperative period of cephalic pancreatic resections greatly increase the risks of the procedure [12]. These aspects could be observed in this case report. Significant venous congestion in the small intestine remained evident after test application despite the preservation of the first jejunal branch of the SMV and an ileal tributary of caliber similar to the one affected by the tumor, occluded in the test.
The venous congestion extension evaluation of the small intestine observed after the occlusion of the compromised vein seems to be the best parameter to define the need of vascular reconstruction. Consequently, the test is indicated to patients with tumor infiltration in any of the SMV tributaries. However, depending on the extension of the small intestine venous congestion, the following protocols can be followed: (i) the resection must be adopted without venous reconstruction in patients with no venous congestion in the small intestine, or when only a small intestinal segment (<30–40 cm) of the congestive proximal jejunum is observed, since the resection of the proximal jejunum is often performed in PD cases; (ii) in those patients with venous congestion of a larger quantity of small intestine in which venous reconstruction of the affected branch is possible, the resection procedure also proceeds with the reconstruction of the recommended vessel; (iii) in those patients in whom venous reconstruction is not possible due to the small size of the vessel, resection of the pancreatic tumor is still possible safely if the part of the congestion small intestine is resected; and (iv) finally, the resection procedure may be aborted when none of the above alternatives is possible.
This case report emphasizes the adoption of an easy, fast and apparently efficient test that may help the decision-making on the intraoperative management of choice for pancreatic cancer cases with SMV tributary tumor infiltration.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- multidetector computed tomography
- weight reduction
- anastomosis, surgical
- intestine, small
- mesenteric vein
- reconstructive surgical procedures
- surgical procedures, operative
- ileum
- neoplasms
- lymph node biopsy
- jaundice, obstructive
- venous occlusion
- pancreatic adenocarcinoma
- pancreas head
- superior mesenteric vein



