-
PDF
- Split View
-
Views
-
Cite
Cite
Naoki Ikari, Akiyoshi Seshimo, Kiyoaki Taniguchi, Sho Kotake, Takuji Yamada, Kosuke Narumiya, Masakazu Yamamoto, Constant maintenance of an alternative route of coronary flow in radical surgery for gastric cancer following coronary artery bypass grafting involving the right gastroepiploic artery: a case report, Journal of Surgical Case Reports, Volume 2017, Issue 6, June 2017, rjx096, https://doi.org/10.1093/jscr/rjx096
Close - Share Icon Share
Abstract
We describe a 64-year-old man diagnosed as having gastric cancer with a patent right gastroepiploic artery (RGEA) used for coronary artery bypass grafting (CABG). Before gastrectomy, the native coronary artery was revascularized to safely dissect the infrapyloric lymphatic tissue along the layer recently identified as an appropriate layer for radical lymphadenectomy, in anticipation of preserving the radically skeletonized RGEA. The perioperative strategy was feasible. Postoperatively, hemorrhage extended the stopping period of antiplatelet therapy. However, since the RGEA was preserved, an alternative route was available for coronary flow. After a 41-month postoperative follow-up, the patient remained in good health, with no recurrence or cardiac ischemia. In this case, the alternative route of coronary flow could be constantly maintained, although radical infrapyloric lymphadenectomy had been performed. Preoperative revascularization and preserving the RGEA with radical skeletonization can be a safe yet permissibly radical strategy for gastric cancer treatment following CABG involving the RGEA.
INTRODUCTION
Gastric cancer following coronary artery bypass grafting (CABG) involving the right gastroepiploic artery (RGEA) still has a risk of metastasis to the infrapyloric lymph nodes (LNs), which encompass the first branch and proximal part of the RGEA [1–4]. Preoperative coronary revascularization enables surgeons to perform radical infrapyloric lymphadenectomy with RGEA resection [4]. However, since the revascularized native coronary artery harbors a risk of stent thrombosis or restenosis [5, 6], a route of coronary flow provided by the RGEA must be maintained even after surgery. Recently, an appropriate layer for the infrapyloric lymphadenectomy has been determined [7]. Thus, integrating this knowledge, we describe the first patient in whom the native coronary artery was revascularized before surgery to safely dissect LNs along the appropriate layer for infrapyloric lymphadenectomy [7], in anticipation of preserving the radically skeletonized RGEA.
CASE REPORT
A 64-year-old man with a history of angina pectoris and thrombocytopenia underwent CABG procedures at the age of 33 and 55 years. During the second CABG, the RGEA was used as a graft for the right coronary artery.
Nine years after the second CABG, he was admitted to our hospital with hematemesis. An upper gastrointestinal endoscopy showed a lesion with a hemorrhagic central ulcerative area and elevated margins that were poorly differentiated from the surrounding tissue on the posterior wall of the antrum. A diagnosis of poorly differentiated adenocarcinoma was confirmed by biopsy. No other apparent metastases to other organs were identified. On the basis of a diagnosis of advanced gastric cancer localized at the gastric angle, we performed distal gastrectomy with D2 LN dissection.
The patient underwent coronary arteriography preoperatively. Based on confirmation of a patent RGEA (Fig. 1a), the stenotic native coronary artery was revascularized (Fig. 1b) using bare-metal stents (BMS) to avoid cardiac ischemia due to intraoperative manipulation of the RGEA (Fig. 1c–e). Definitive gastric surgery was performed 4 weeks after revascularization. The location of the RGEA was easily confirmed intraoperatively (Fig. 2a). Indigo carmine dye was administered into the greater curvature of the gastric angle, adjacent to the tumor, to observe the lymph stream, through which the infrapyloric lymph ducts could also be observed (Fig. 2b). We detached the adipose tissue bearing lymphatic tissue along the outermost layer of nerves that twine around the surface of the pancreas and anterior superior pancreaticoduodenal vein [7] (Fig. 2c). After the lymphatic tissue was removed along the outermost layer of nerves, the remaining skeletonized RGEA was preserved (Fig. 2d). Histopathologically, the tumor was 35 × 25 mm2, type 3, por2, pT3, pN0. Heparin treatment was initiated on postoperative day (POD) 1. However, it was interrupted from PODs 6 to 13 because of a transient hematic discharge from the drainage tube, and antiplatelet therapy was reinitiated on POD 15. The patient was discharged on POD 18. Over the 41-month follow-up period, he did not experience any recurrence of the gastric cancer or cardiac ischemia.
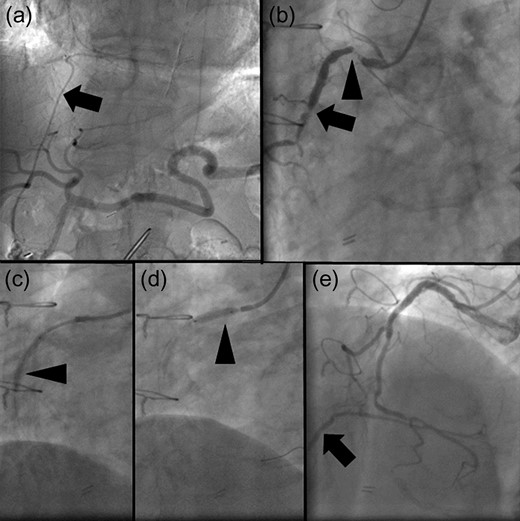
(a) Angiogram of the celiac artery showing a patent right gastroepiploic artery (RGEA) (arrow). (b) Coronary angiogram showing severe stenosis of the native right coronary artery (RCA). The proximal and middle portions of the RGEA exhibit 99% (arrowhead) and 90% (arrow) stenosis. (c, d) Two bare-metal stents (BMS) are implanted in the native RCA (arrowheads). (e) When the RCA is revascularized using the BMS, the restricted blood flow improved. Moreover, the retrograde flow of contrast medium to the patent RGEA is observed (arrow).
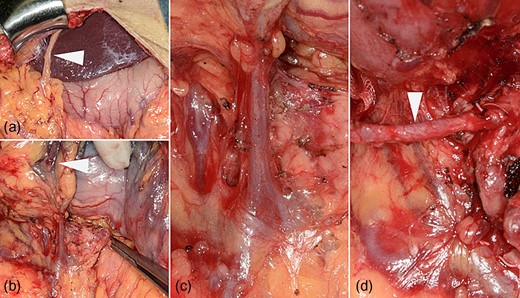
(a) The right gastroepiploic artery (RGEA) at the start of the surgery (arrowhead). (b) Lymph flow from the gastric angle is observed in the infrapyloric lymph ducts using indigo carmine dye (arrowheads). (c) The translucent, whitish autonomic nerves that twine around the surface of the pancreas and the anterior superior pancreaticoduodenal vein can be observed during dissection along the outermost layer of nerves. (d) The RGEA is preserved after infrapyloric lymphadenectomy (arrow).
DISCUSSION
According to the Japanese Gastric Cancer Association guidelines, dissection of infrapyloric LNs, which encompass the first branch and proximal part of the RGEA, is required for distal and total D1 and D2 gastrectomies for gastric cancer [1, 2]. Although CABG using the RGEA may lead to certain changes in the lymph stream, metastasis to the infrapyloric LNs is common among patients with gastric cancer even after CABG using the RGEA [3, 4]. In our case, there were no swollen LNs. However, we confirmed that the lymph stream from the gastric antrum toward the infrapyloric area was maintained (Fig. 2b), which suggested the need for preventive infrapyloric lymphadenectomy.
Conventionally, coronary revascularization was performed to conduct infrapyloric lymphadenectomy with RGEA resection [4]. However, considering the risk of stent thrombosis or restenosis of the revascularized native coronary artery [5, 6], a route of coronary flow provided by the RGEA must be maintained even after surgery. Generally, stent thrombosis has been reported in 1% of cases of coronary artery stenting and is associated with a mortality rate of 20–45% [5]. The 12-month rate of clinical restenosis, defined by target-lesion revascularization, is estimated at 20% in cases when BMSs have been used [6].
Recent advances in understanding the topographic anatomy of the infrapyloric lymph region have helped determine the appropriate layer for infrapyloric lymphadenectomy: the outermost layer of nerves around the surface of the pancreas and major vessels [7]. Thus, in the present case, we did not perform preoperative coronary revascularization to resect the RGEA; instead, we did it to safely remove the lymphatic tissue dissecting along the outermost layer of nerves, in anticipation of preserving the radically skeletonized RGEA.
As a result, performing dissection along the outermost layer of nerves was technically feasible even after CABG involving the RGEA. In addition, although we experienced a postoperative complication that delayed restarting antiplatelet therapy, we were able to provide an alternative route of coronary flow during this period, since the patent RGEA was preserved intraoperatively. Longitudinally, the patient did not experience any recurrence of the gastric cancer or cardiac ischemia.
There are two limitations of our report. The oncological difference between resecting and preserving the RGEA through radical skeletonization is still uncertain. The long-term patency of the inferior route of coronary flow is also uncertain. However, our surgical strategy for gastric cancer after CABG involving the RGEA could be useful considering its technical feasibility, perioperative safety, curability of lymphadenectomy before preserving the RGEA, and longitudinal outcome with no recurrence or cardiac ischemia over the 41-month follow-up period.
A series of coronary revascularization, radical infrapyloric lymphadenectomy along the outermost layer and preservation of the skeletonized RGEA could be a well balanced strategy fulfilling both permissible curability and safety under the restricted conditions of gastric cancer after CABG involving the RGEA.
AUTHORS’ CONTRIBUTIONS
N.I. performed the acquisition, investigation and analysis of data, and wrote the article. K.T., S.K. and T.Y. acquired the data. A.S. and K.N. performed the investigation and analyzed the data. A.S. and M.Y. helped in article writing. All authors read and approved the final manuscript.
ACKNOWLEDGMENTS
We would like to thank Dr Kamishima (Department of Cardiology) for his expertise. We would also like to thank Editage (www.editage.jp) for English language editing.
CONFLICT OF INTEREST STATEMENT
None declared.
CONSENT FOR PUBLICATION
Written informed consent was obtained from the patient for publication of this Case report and any accompanying images.
REFERENCES
- antiplatelet agents
- myocardial ischemia
- coronary artery bypass surgery
- coronary artery
- hemorrhage
- gastrectomy
- gastric cancer
- drug administration routes
- follow-up
- gastroepiploic artery
- lymph node excision
- lymphoid tissue
- personal satisfaction
- preoperative care
- surgical procedures, operative
- lymph node dissection
- revascularization
- radical excision of lymph nodes
- patents
- fluid flow



