-
PDF
- Split View
-
Views
-
Cite
Cite
Aditi Halder, Artiene Tatian, A. Cristina Vargas, Rooshdiya Karim, Mark Latt, Cutaneous presentation of gastrointestinal adenocarcinoma, Journal of Surgical Case Reports, Volume 2017, Issue 6, June 2017, rjx083, https://doi.org/10.1093/jscr/rjx083
Close - Share Icon Share
Abstract
Seeding of a central nervous system malignancy to the abdominal cavity is an uncommon but well documented complication of a ventriculoperitoneal (VP) shunt. However, the metastasis of a primary gastrointestinal cancer to the skin via a VP shunt is extremely rare. We report the clinical case of an 85-year-old male who presented with a right upper quadrant nodule over his shunt, which on histopathology and tumour marker profile was diagnosed as an adenocarcinoma of likely upper gastrointestinal origin. This case illustrates the importance of proceeding to biopsy to inform prognosis and management, despite the risks of shunt infection.
INTRODUCTION
The dissemination of a primary central nervous system malignancy to the abdomen is a recognized potential complication of a ventriculoperitoneal (VP) shunt [1, 2]. However, the retrograde seeding of a primary gastrointestinal cancer to the dermis along a VP shunt is extremely rare. We report a case where internal malignancy was diagnosed following investigation of a subcutaneous mass overlying a VP shunt.
CASE REPORT
An 85-year-old male presented with increasing pain in the right upper quadrant and a skin lump since the insertion of a VP shunt 9 months prior to treat normal pressure hydrocephalus. Anaemia [Hb 7.7 g/dL (13–17)], malaise, reduced appetite and 5 kg weight loss over 5 months were also elucidated on further history, although per rectal bleeding or darkened stools were absent.
Examination of the abdomen revealed a tender, violaceous subcutaneous nodule with epidermal changes overlying the shunt (Fig. 1). Multiple smaller subcutaneous nodules without epidermal changes were also noted to track along the shunt, including a prominent lesion at the level of the right clavicle (Fig. 2).
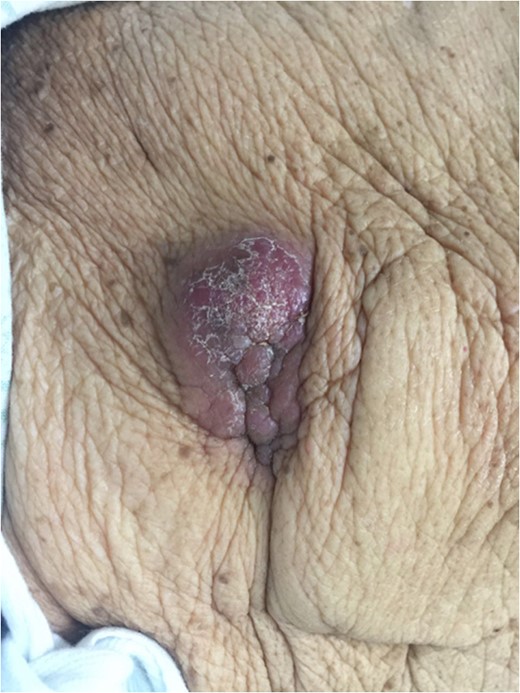
Right upper quadrant lesion overlying intra-abdominal insertion site of ventriculoperitoneal shunt.
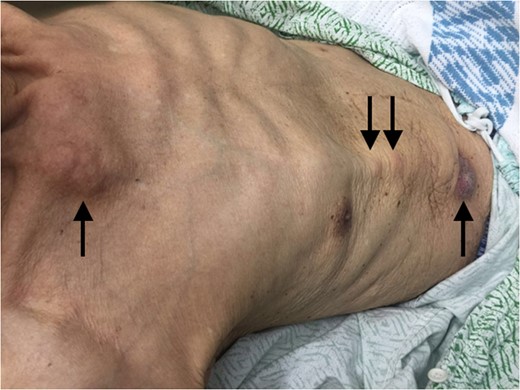
Multiple subcutaneous nodules tracking along the ventriculoperitoneal shunt.
A CT scan of the abdomen and pelvis 2 months prior to hospital admission found the right upper quadrant lesion most likely attributable to scar tissue, of clinically indeterminate significance. Sonography of the right upper quadrant and sternal lesions found echogenic soft tissue masses of similar appearance surrounding the VP shunt. Accepting the risk of infection, a biopsy of the main abdominal lesion was performed.
On histology, an intradermal malignant neoplasm with features of adenocarcinoma was identified overlying the VP shunt (Fig. 3). Immunohistochemistry was non-specific for the primary site, with a primary adnexal origin unable to be excluded on histology alone (Table 1). However, with positive CK19 and mCEA, negative D2-40 and CK5/6, as well as elevated tumour markers [Ca19.1 1545 kU/L (≤37), Ca125 181 kU/L (0–35), CEA 19.1 μg/L (0–3.0)], the diagnosis favoured metastatic adenocarcinoma, most likely from the upper gastrointestinal tract, rather than a primary cutaneous adnexal adenocarcinoma.
| Immunostain . | Result . |
|---|---|
| CK cocktail | Positive |
| CK19 | Positive |
| EMA | Positive |
| CDX2 | Positive |
| P63 | Patchy positive stain |
| mCEA | Patchy positive stain with predominant luminal apical stain |
| B-catenin | Positive (strong membranous stain with no nuclear stain seen) |
| CK20 | Negative |
| CK7 | Negative |
| PSA | Negative |
| SMA | Negative |
| GCDFP15 | Negative |
| Calretinin | Negative |
| HBME-1 | Negative |
| TTF1 | Negative |
| ER | Negative |
| PR | Negative |
| Ki-67 | High (>60%) |
| D2-40 | Negative |
| CK5/6 | Negative |
| Immunostain . | Result . |
|---|---|
| CK cocktail | Positive |
| CK19 | Positive |
| EMA | Positive |
| CDX2 | Positive |
| P63 | Patchy positive stain |
| mCEA | Patchy positive stain with predominant luminal apical stain |
| B-catenin | Positive (strong membranous stain with no nuclear stain seen) |
| CK20 | Negative |
| CK7 | Negative |
| PSA | Negative |
| SMA | Negative |
| GCDFP15 | Negative |
| Calretinin | Negative |
| HBME-1 | Negative |
| TTF1 | Negative |
| ER | Negative |
| PR | Negative |
| Ki-67 | High (>60%) |
| D2-40 | Negative |
| CK5/6 | Negative |
CK, cytokeratin; EMA, epithelial membrane antigen; CDX ,caudal type homeobox; mCEA, monoclonal carcinoembryonic antigen; PSA, prostate specific antigen; SMA, smooth muscle actin; GCDFP, gross cystic disease fluid protein; HBME, Hector Battifora mesothelial; TTF, thyroid transcription factor; ER, oestrogen receptor; PR, progesterone receptor.
| Immunostain . | Result . |
|---|---|
| CK cocktail | Positive |
| CK19 | Positive |
| EMA | Positive |
| CDX2 | Positive |
| P63 | Patchy positive stain |
| mCEA | Patchy positive stain with predominant luminal apical stain |
| B-catenin | Positive (strong membranous stain with no nuclear stain seen) |
| CK20 | Negative |
| CK7 | Negative |
| PSA | Negative |
| SMA | Negative |
| GCDFP15 | Negative |
| Calretinin | Negative |
| HBME-1 | Negative |
| TTF1 | Negative |
| ER | Negative |
| PR | Negative |
| Ki-67 | High (>60%) |
| D2-40 | Negative |
| CK5/6 | Negative |
| Immunostain . | Result . |
|---|---|
| CK cocktail | Positive |
| CK19 | Positive |
| EMA | Positive |
| CDX2 | Positive |
| P63 | Patchy positive stain |
| mCEA | Patchy positive stain with predominant luminal apical stain |
| B-catenin | Positive (strong membranous stain with no nuclear stain seen) |
| CK20 | Negative |
| CK7 | Negative |
| PSA | Negative |
| SMA | Negative |
| GCDFP15 | Negative |
| Calretinin | Negative |
| HBME-1 | Negative |
| TTF1 | Negative |
| ER | Negative |
| PR | Negative |
| Ki-67 | High (>60%) |
| D2-40 | Negative |
| CK5/6 | Negative |
CK, cytokeratin; EMA, epithelial membrane antigen; CDX ,caudal type homeobox; mCEA, monoclonal carcinoembryonic antigen; PSA, prostate specific antigen; SMA, smooth muscle actin; GCDFP, gross cystic disease fluid protein; HBME, Hector Battifora mesothelial; TTF, thyroid transcription factor; ER, oestrogen receptor; PR, progesterone receptor.
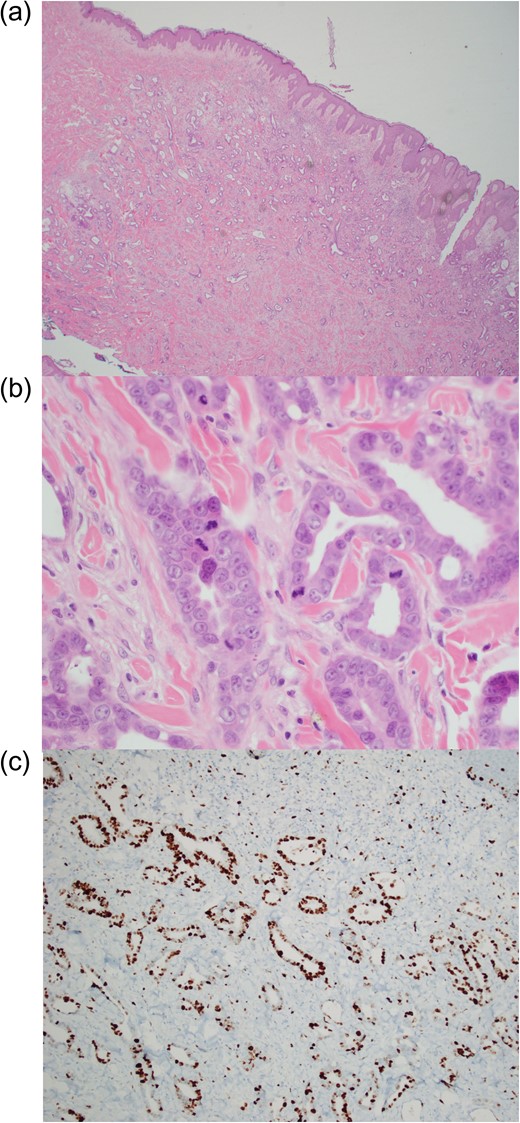
Biopsy sample demonstrating (a) dermal proliferation of infiltrating malignant glands (haematoxylin and eosin stain, ×20 magnification), (b) marked nuclear atypic and mitotic activity (haematoxylin and eosin stain, ×400 magnification) and (c) pancytokeratin stain of adenocarcinoma (×40 magnification).
Given our patient’s frailty and age, it was determined he was not a suitable candidate for chemotherapy or operative management of his adenocarcinoma. The decision was made to avoid a further CT–PET scan as it would not alter management. He was discharged home with services in place for palliative management, and for future review by his neurosurgeon for shunt level adjustment or possible shunt resiting.
DISCUSSION
The role of a VP shunt in facilitating metastatic spread of central nervous system cancers is uncommon but acknowledged. Various primary brain tumours have been reported to metastasize to the abdominal cavity via a VP shunt [1, 2]. However, seeding of gastrointestinal carcinoma along a VP shunt is exceptionally rare. Only one case has been previously documented in the literature, in a 61-year-old patient with normal pressure hydrocephalus who was found to have an abdominal metastatic lesion secondary to aggressive pancreatic carcinoma [3]. Our patient is the first reported case of presumed multiple skin metastases along a VP shunt from a gastrointestinal source of adenocarcinoma.
Carcinoma surrounding a VP shunt poses a risk of obstruction secondary to tumour enlargement. Obstruction of a VP shunt has been reported in a patient with breast cancer that metastasized to and surrounded the shunt [4]. Ongoing monitoring for ventricular enlargement may be required, with neurosurgical input to determine if the shunt requires resiting or replacement in the future.
In summary, this case illustrates the importance of considering malignancy in the differential diagnosis of a nodule overlying a VP shunt, with thorough history and examination essential to diagnosis. Although invasive, biopsy of the lesion proved key to informing prognosis and future management of our patient.
ACKNOWLEDGEMENTS
We would like to kindly acknowledge Dr Patricia Lowe’s dermatology team for their review of our patient and suggestion to proceed to biopsy.



