-
PDF
- Split View
-
Views
-
Cite
Cite
Szu-Kai Hsu, Chih-Ju Chang, I-Chang Su, Rendering a ruptured arteriovenous malformation more susceptible to spontaneous obliteration as a possible treatment strategy for cerebral AVM, Journal of Surgical Case Reports, Volume 2017, Issue 4, April 2017, rjx073, https://doi.org/10.1093/jscr/rjx073
Close - Share Icon Share
Abstract
Spontaneous regression of cerebral arteriovenous malformation (AVM) is a rare phenomenon, but its occurrence is an important consideration in treatment planning. A 58-year-old male was found to have a high-flow AVM of Spetzler–Martin Grade III. Before his scheduled treatment, the AVM ruptured with a large parenchymal hemorrhage. Following emergency decompressive surgery, a targeted embolization procedure was performed to obliterate the ruptured weak point and to reduce the shunting flow. The residual AVM became a malformation harboring angio-architectural factors favoring spontaneous obliteration. Together with other favorable clinical factors, including prior parenchymal hemorrhage and neurosurgical intervention, the residual AVM spontaneously regressed in 2 months. This case highlighted a possible treatment strategy in that, for a ruptured AVM in which definite treatment is not possible, an alternative is to treat the AVM into a situation in which as many favorable factors as possible for spontaneous AVM regression are present.
U
Cerebral arteriovenous malformation (AVM) is a heterogeneous cerebrovascular disease in which the clinical manifestation varies widely. While most AVMs require treatment when they become symptomatic or even rupture [1], other AVMs might regress spontaneously without any treatment or following partial treatment [2].
The phenomenon of spontaneous AVM regression is multifactorial, but the relationships between various factors remain complex and controversial. Whatever the cause, the importance of this rare phenomenon cannot be over-emphasized, because its occurrence is a consideration in treatment planning for cerebral AVM.
Herein, we report a case in which a ruptured cerebral AVM regressed after the patient received emergency decompressive surgery and targeted embolization of the AVM. We analyzed possible contributing factors to this phenomenon, and proposed our experience in this case as the basis for an alternative treatment strategy for a ruptured AVM when definite treatment is not possible.
CASE REPORT
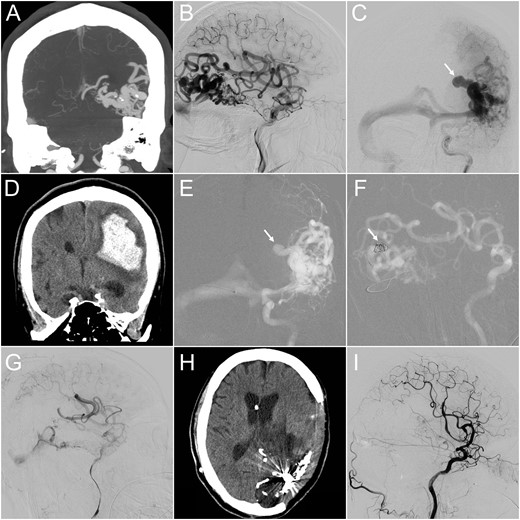
(A) CT angiography (coronal view) revealed a 4-cm AVM at the left temporo-occipital region. (B and C) Lateral projection of early (B) and frontal projection of late (C) arterial phase of left internal carotid angiograms demonstrated a high-flow AVM and a co-existing venous aneurysm on the draining vein (arrow), which ruptured 1 month after this angiographic study. (D) Emergency CT performed after the patient lost consciousness revealed a massive parenchymal hemorrhage at the left parieto-temporal region. (E and F) Microcatheter was advanced into the ruptured venous aneurysm, where detachable coils were deployed (arrow). (G) Following targeted embolization, the size and flow of the residual AVM were significantly reduced. (H) Follow-up CT 3 months later showed total resolution of the brain edema. (I) The follow-up internal carotid angiogram also demonstrated complete angiographic regression of the residual AVM.
DISCUSSION
Spontaneous AVM regression is a rare event [3]. It has been postulated that this phenomenon is closely correlated with various clinical or radiological features. For example, AVM with hemorrhagic presentation has been shown to be related to this phenomenon [2, 3]. Possible causes include ‘mass effects’ or ‘brain swelling’ secondary to parenchymal hemorrhage. Either cause will result in kinking or even occlusion of the vasculature related to the AVM, and these anatomical and hemodynamic disturbances might subsequently lead to nidus obliteration.
AVM size and the number of arterial feeders are also important. Previous reports have demonstrated that 93% of AVMs with spontaneous regression are smaller than 2 cm [4], and there are significantly fewer feeding arteries in AVMs that undergo spontaneous regression [3].
AVMs harboring only a single draining vein have also been shown to have a greater chance of undergoing spontaneous regression than those with multiple draining veins [2]. A possible explanation for this is that venous outflow obstruction can lead to flow stasis inside the AVM nidus, and obstruction of a single vein is more likely to occur than obstruction of multiple draining veins [4]. This hypothesis appears somewhat contradictory to the previous observation in that venous outlet restriction is actually a known risk factor for AVM rupture [5]. However, when the restriction process is gradual, and there is a concomitant reduction of AVM flow, the overall hemodynamic changes might favor flow stasis inside the AVM nidus, leading to AVM obliteration.
Regarding our case, the original AVM was large, the shunting flow was high, and there were multiple arterial feeders and a single major draining vein. The overall angio-architecture not only did not favor spontaneous regression, but also warranted aggressive treatment. However, after the AVM ruptured, the parenchymal hemorrhage caused significant brain edema and mass effects on the whole AVM complex, including the feeding arteries, nidus and single draining vein. Our embolization procedure further converted this AVM into a malformation of significantly smaller size, fewer arterial feeders and a smaller shunting flow. Therefore, in combining multiple favoring factors, including the patient’s clinical presentation (i.e. hemorrhage and brain edema), pre-existing angio-architectural factors (i.e. a single draining vein), and post-procedural factors (i.e. post-embolization flow reduction), the AVM was converted to a hemodynamic status more susceptible to regression. Over a period of time, the whole AVM complex, including the venous outflow, the remaining arterial feeders and the AVM nidus, gradually underwent thrombosis and regression. Finally, the AVM completely regressed, and additional definite treatment was no longer necessary.
This case study may change our perception and broaden our considerations in treatment planning for AVM. Neurosurgical excision and radiosurgery, with or without adjuvant embolization, are the two standard modalities for definite treatment of cerebral AVM. However, when the clinical conditions or radiological characteristics of the AVM preclude the possibility of definite treatment, targeted treatment of the AVM is considered a better option, in order to reduce AVM-related risks [6]. As seen in our case, the initial strategy of definite treatment for the patient was changed to a life-saving strategy after when life-threatening hemorrhage occurred. Although this strategy left a residual AVM behind, it created a less-risky clinical environment that allowed the patient to recover gradually without delay. Furthermore, as most of the factors favoring spontaneous AVM regression were present, we were able to adopt a wait-and-see policy in terms of its possible occurrence without posing additional risks to the patient. We, therefore, hypothesized that rendering the ruptured AVM more susceptible to spontaneous obliteration by targeted embolization of the AVM was an alternative treatment consideration, especially when definite treatment was not possible or not suitable. Of course, angiographic follow-up at regular intervals was mandatory in order to confirm AVM obliteration and to ensure that the AVM did not recanalize [7].
In conclusion, we postulate that, in certain circumstances, when definite treatment for a ruptured AVM is too risky, an alternative strategy is to treat the AVM to result in a hemodynamic status that fulfills as many as possible factors favorable for spontaneous AVM regression.
ACKNOWLEDGEMENTS
None.
CONFLICT OF INTEREST STATEMENT
The authors declare that there is no conflict of interest regarding the publication of this paper.



