-
PDF
- Split View
-
Views
-
Cite
Cite
Yohei Kawatani, Hirotsugu Kurobe, Yoshitsugu Nakamura, Yuji Suda, Yoshinori Okuma, Shinichiro Sato, Toru Hashimoto, Takaki Hori, Acute pancreatitis caused by pancreatic ischemia after TEVAR combined with intentional celiac artery coverage and embolization of the branches of the celiac artery, Journal of Surgical Case Reports, Volume 2017, Issue 2, February 2017, rjx029, https://doi.org/10.1093/jscr/rjx029
Close - Share Icon Share
Abstract
Covering and embolizing the celiac artery has been reported to be a relatively safe procedure, owing to the rich collateral pathway between the celiac artery and superior mesenteric artery. A 69-year-old man presented with an aneurysm on the distal descending aorta. The proximity of the aneurysm to the celiac artery origin necessitated covering the artery with a stent graft. Additionally, the celiac trunk was short, increasing the risk for Type II endoleak. The origin of the celiac artery was covered after embolization of the branches of the celiac artery. Postoperatively, nausea and abdominal pain appeared, and the amylase level and white blood cell count were elevated. Contrast-enhanced computed tomography and abdominal ultrasonography revealed necrosis and cyst formation in the pancreatic tail, resulting in a diagnosis of acute pancreatitis caused by pancreatic ischemia. Conservative treatment was applied. After discharge, although walled-off necrosis remained, the patient was doing well, without any clinical symptoms.
INTRODUCTION
Endovascular stent graft implantation is a minimally invasive operation with a low mortality rate, low complication incidence, short hospitalization period [1], and good short- and mid-term results [2]. However, it has some anatomical restrictions, requiring landing zones. If the distance between the aneurysm and aortic branch is short, conventional methods cannot be used. The potential for endovascular stent graft implantation can be expanded by covering branches to extend the landing zone. Especially, covering the celiac artery has a low complication risk, as there is often an arcade with the superior mesenteric artery (SMA) [3].
CASE PRESENTATION
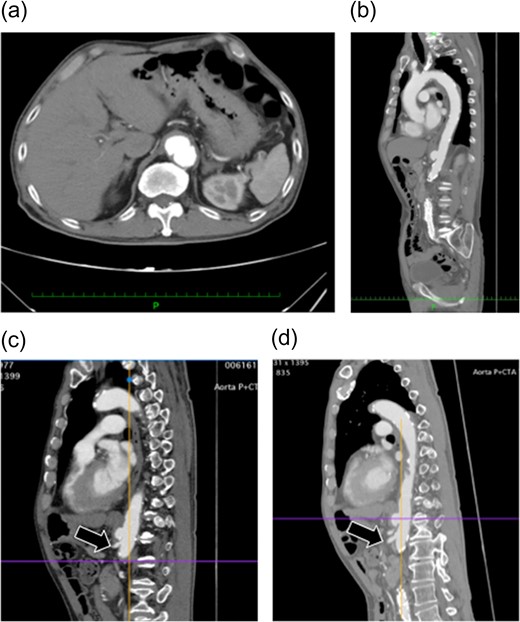
Preoperative enhanced computed tomography. (a and b) A saccular aneurysm can be observed on the descending aorta. (c) The aneurysm is very close to the celiac artery (arrow: origin of the celiac artery). The length of the celiac trunk is 11 mm. (d) The distance to the SMA is 21 mm (arrow: origin of the SMA). No endoleaks were noted in the aneurysm.
OPERATION
The SMA was cannulated and selectively imaged. Communication between the SMA and peripheral celiac artery was observed via the gastroduodenal artery. Selective coil embolization was performed in the common hepatic, splenic and right gastric arteries. The coils were placed centrally to preserve the gastroduodenal and proper hepatic arteries.
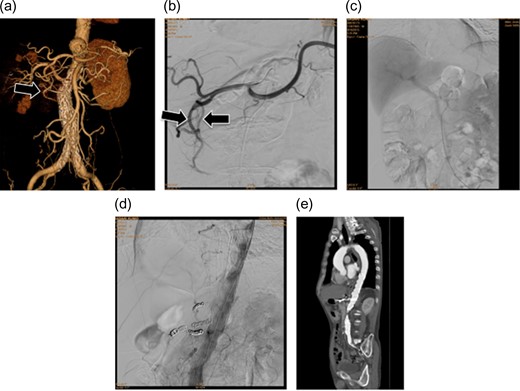
(a) Preoperative three-dimensional computed tomography (3D-CT) angiography showing the aneurysm. An arcade for the celiac and superior mesenteric arteries was confirmed via the gastroduodenal artery (arrow). (b) Intraoperative image of the celiac artery. As on the 3D-CT images, an arcade via the gastroduodenal artery was confirmed (arrow). (c) Portal phase image of the SMA. The liver is imaged during blood flow from the portal vein, confirming that the liver will be supplied with blood even if the hepatic artery blood flow decreases. (d) Angiography after placing the stent graft. No endoleaks, including Type II endoleaks from the celiac artery, are observed. Accordingly, the treatment was deemed effective. (e) Postoperative contrast-enhanced CT. No endoleaks are observed, so the treatment was considered successful.
POSTOPERATIVE COURSE
Immediately after the operation, the patient complained of nausea and vomiting.
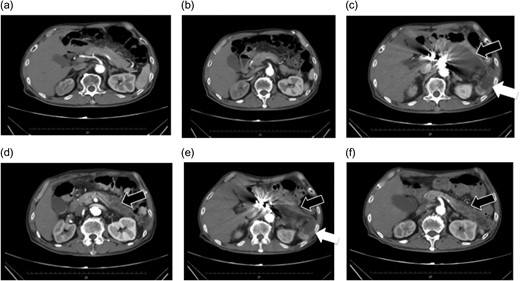
(a and b) Preoperative contrast-eCT. (c and d) eCT performed on postoperative day 13. A cyst is seen on the pancreatic tail (black arrow), while no contrast effect is seen in the spleen (white arrow). (e and f) CT performed on postoperative day 21. The pancreatic cyst has started to shrink. Part of the spleen becoming necrotic does not conflict with an etiology of ischemia due to embolization of the splenic artery.
Intravenous hydration was initiated. His leukocyte count rose to 31 290 µl–1 on postoperative day 8, after which it gradually improved, normalizing on postoperative day 21. Similarly, CRP peaked at 24.55 mg/dl on postoperative day 7, after which it gradually improved, plateauing at around 2.0 mg/dl.
Follow-up eCT on postoperative day 13 showed expanded necrotic and cystic areas compared to immediately after onset. Infected necrosis was suspected. Meropenem 1.0 g/day and a protease inhibitor, gabexate mesilate 2500 mg/day, were administered. eCT on postoperative day 20 showed shrinkage of the pancreatic necrosis and cyst.
The patient's symptoms and physical signs improved by postoperative day 24, and he was discharged on postoperative day 40. At the follow-up, no exacerbation of his clinical signs was noted.
DISCUSSION
Gallstones are considered the most common cause of pancreatitis (40–70%), followed by alcoholism (25–35%), while more rare causes include hypercalcemia, hypertriglyceridemia, drugs [4] and pancreatic ischemia occurring after pancreas implant surgery [5].
The patient did not have preoperative risk factors for pancreatitis. Additionally, CT images taken at the arterial and late phases (180 s) did not show a contrast effect in the splenic artery distal to the embolism or the spleen. Pancreatitis occurred immediately after the arteries perfusing the pancreas were surgically embolized. Therefore, we diagnosed pancreatitis due to reduced pancreatic blood flow secondary to embolization (Fig. 2).
Ischemic acute pancreatitis is extremely rare. A review by Thilo et al. found only 11 cases that occurred after intraoperative cardiopulmonary bypass, celiac artery thromboembolism or systemic hypoperfusion due to shock. Moreover, the clinical presentation and examination findings of acute pancreatitis resemble those of other forms of pancreatitis. However, ischemic acute pancreatitis often follows a more serious course than acute pancreatitis from other causes, with a high mortality rate (64%) [5].
Ligation, dissection and embolization of the celiac artery are considered relatively safe because of the communication between the branches of the celiac artery and SMA [6, 7]. Although rare, complications due to reduced blood flow, such as gastric and duodenal ulcers, ischemic cholecystitis, liver dysfunction and splenic necrosis, have been reported [8].
We confirmed the collateral pathways on preoperative CT, and imaged the SMA intraoperatively before embolizing the celiac artery, confirming communication between the branches (Fig. 2). Nevertheless, pancreatic ischemia occurred. This patient's collateral pathway through the dorsal splenic artery was considered insufficient to perfuse the pancreatic tail and spleen. Additionally, this patient had a history of endovascular aortic repair. It is possible that this previous procedure could have had adverse effects on the perfusion of the celiac artery via the SMA. If we had performed SMA arteriography at the beginning of the surgery with the celiac artery occluded by balloon, we may have been able to predict and avoid these phenomena. We should consider carefully which arteries to be embolized.
Limiting embolization to the root as much as possible is beneficial for maintaining peripheral collateral circulation. However, this area in the celiac artery was short, and placing a coil in the celiac trunk was not sufficient for embolization. Furthermore, residual blood flow in the celiac artery causes major Type II endoleak. Leon et al. examined 25 cases undergoing intentional celiac coverage and found one in which death was thought to have occurred from a Type II endoleak [8]. Therefore, we thought that its branches should be embolized.
The treatment of ischemic acute pancreatitis is basically similar to that for regular acute pancreatitis. But a therapeutic effect can be obtained in this etiology by performing revascularization to improve the ischemia, along with necrosectomy via laparotomy or drainage through a minimally invasive approach (laparoscopically or CT-guided puncture). However, pancreatic cysts are fragile in the initial stages, so the risk of surgical complications is hence high. Thus, surgery should be considered carefully. Low-dose intravenous heparin is sometimes administered in ischemic pancreatitis for thrombolysis and to maintain microcirculation [5]. In our case, however, the embolism was not due to a thrombus, but rather from a coil. We thus decided against heparin administration.
ACKNOWLEDGEMENTS
Consent to publication was obtained from the patient. This case has been presented and discussed at the International Conference on Complications in Interventional Radiology 2016 (ICCIR 2016) chaired by the Cardiovascular and Interventional Radiological Society of Europe.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- pancreatitis, acute
- ischemia
- abdominal pain
- computed tomography
- descending aorta
- aneurysm
- embolization
- amylases
- celiac artery
- cysts
- leukocyte count
- superior mesenteric artery
- nausea
- necrosis
- diagnosis
- pancreas
- abdominal ultrasonography
- endoluminal grafts
- pancreas tail
- endoleak
- thoracic endovascular aortic repair
- conservative treatment



