-
PDF
- Split View
-
Views
-
Cite
Cite
Satoshi Washino, Tsuzumi Konishi, Kimitoshi Saito, Masashi Ohshima, Yuhki Nakamura, Tomoaki Miyagawa, Two cases of somatic-type malignancy as a very late relapse of testicular cancer successfully managed by surgical resection, Journal of Surgical Case Reports, Volume 2017, Issue 11, November 2017, rjx233, https://doi.org/10.1093/jscr/rjx233
Close - Share Icon Share
Abstract
A late-relapse germ cell tumor might contain malignant non-germ cell tumors, known as ‘somatic-type malignancy (SM)’. The development of a secondary SM is rare, and this phenomenon remains poorly understood. Case 1 developed lung metastasis 13 years after chemotherapy followed by retroperitoneal lymph node dissection for stage IIA non-seminoma. The tumor increased in size after chemotherapy. The patient underwent a pneumonectomy. Pathology revealed an adenocarcinoma with immature teratoma. The patient has experienced no relapse for 9 years. Case 2 developed a pelvic tumor after 10 years of surveillance for stage I seminoma. The tumor increased in size after chemotherapy. The patient underwent pelvic tumor resection with cystectomy. Pathology revealed a mature teratoma with SMs consisting of sarcoma and adenocarcinoma. The patient has experienced no relapse for 6 months. Surgical resection played a major role in the treatment of very late-relapse germ cell tumors with SM.
INTRODUCTION
A successful multidisciplinary approach for the management of germ cell tumors (GCTs) has resulted in an overall survival rate of >90%. However, 10–30% of patients experience recurrence after the initial treatment [1]. Recently, it has become apparent that a small number of patients experience late relapse, which is defined as the recurrence of a GCT or GCT-derived neoplasm more than 2 years after complete remission (CR) with no evidence of a second primary neoplasm. Very late relapse (>5 years after diagnosis) is even rarer. Tumors with late relapse might contain malignant non-GCTs, which are known as ‘somatic-type malignancies (SMs)’ [2]. However, the optimal therapeutic strategy for SM has not been established because of the rarity of SM.
CASE REPORT
Case 1
A 39-year-old man presented to our institution because of a lung mass identified during a medical check-up. He had a history of a testicular tumor 13 years ago. He underwent a left radical orchiectomy and was diagnosed as embryonal carcinoma of testis, T1N2M0S1 (stage IIA). The patient received GCT-oriented chemotherapy followed by retroperitoneal lymph node dissection (RPLND) for the residual lymph node tumor. The pathology report described an immature teratoma. The patient was followed for 5 years after chemotherapy, and he had no relapse during the follow-up period.
Thirteen years after the first treatment, the patient presented to our institution because of a lung mass detected during a medical checkup. Computed tomography (CT) showed a 76 × 38 mm2 mass in the right lung (Fig. 1A); no other tumor was detected. The alpha-1 fetoprotein (AFP), beta-subunit of human choriongonadotropin (βHCG) and human choriongonadotropin (HCG) levels were 154.2 ng/mL, <0.1 ng/mL and <1.0 mIU/mL, respectively. Late relapse of testicular cancer was highly suspected. After three cycles of GCT-oriented chemotherapy CT showed that the tumor had increased in size (Fig. 1B). He received a pneumonectomy. Pathology showed a well-differentiated adenocarcinoma (Fig. 2A) with immature teratoma (Fig. 2B). The patient was diagnosed with testicular tumor/teratoma with SM. The patient has experienced no relapse for 9 years after pneumonectomy to date.
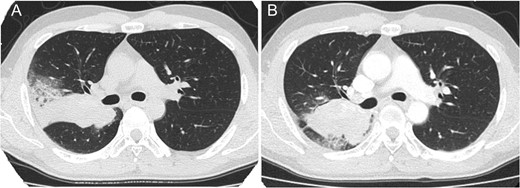
Thoracic computed tomography showing lung tumor before chemotherapy (A) and after chemotherapy (B) in case 1. The tumor increased in size after chemotherapy.
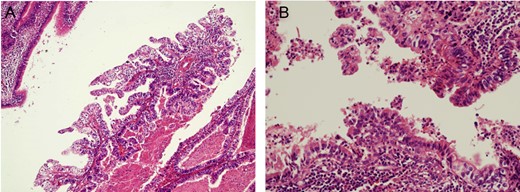
Pathological findings of pneumonectomy specimen in case 1 (H&E): there were components of adenocarcinoma (A, original magnification 200-fold) and immature teratoma (B, original magnification 400-fold).
Case 2
A 58-year-old man presented to our department because of lower abdominal pain. He has a history of testicular cancer from 10 years ago. He underwent a left radical orchiectomy and was diagnosed as seminoma of testis, T1N0M0S0 (stage IA). The patient received no additional therapy.
Ten years after the first treatment the patient presented to our department with a complaint of lower abdominal pain. MRI identified a 10 cm mass in the pelvis that had invaded the bladder (Fig. 3A); no other tumor was detected by thoracic and abdominal CT. The AFP, βHCG, neuron specific enolase and HCG levels were 654.9 ng/mL, 1.2 ng/mL, 36.6 ng/mL and 18.8 mIU/mL, respectively. He underwent a transrectal tumor biopsy, and pathological findings identified a GCT consisting of seminoma, teratoma and sarcoma. The patient was diagnosed with late-relapse testicular tumor/teratoma with SM.
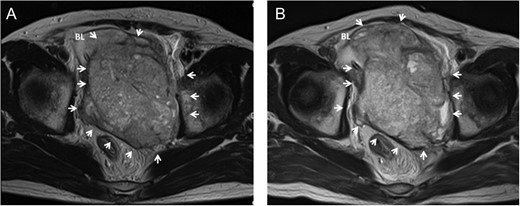
Pelvic MRI showing a large pelvic mass invading to bladder before chemotherapy in case 2 (A). The mass increased in size accompanied with worsening of bladder invasion after chemotherapy (B). Arrow, tumor; BL, bladder.
After one cycle of GCT-oriented chemotherapy the tumor had increased in size (Fig. 3B). He underwent tumor resection and cystectomy. Pathological findings revealed a mature teratoma with SM, consisting of rhabdomyosarcoma, glioblastoma and adenocarcinoma, that had invaded the bladder; no seminoma component was found (Fig. 4). The levels of all tumor markers decreased to their normal ranges after surgery. To date, the patient has experienced no relapse for 6 months.
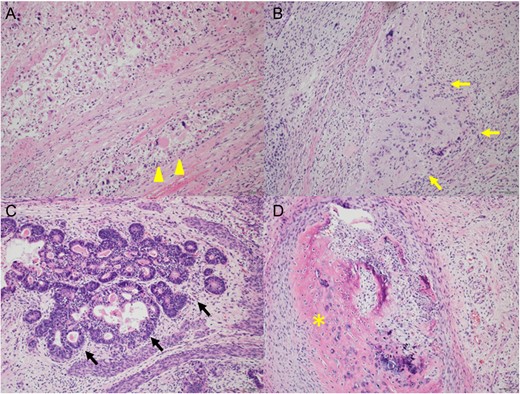
Pathological findings of resected tumor in case 2 (H&E, original magnification 200-fold); there were mixed tumor, consisting of rhabdomyosarcoma (A, arrowhead), glioblastoma (B, yellow arrow), adenocarcinoma (C, black arrow) and mature teratoma (D, asterisk).
DISCUSSION
The introduction of cisplatin-based chemotherapy for the treatment of testicular GCTs transformed testicular cancer into a curable neoplasm. However, relapse occurs in 10–30% of patients after the initial treatment. Although relapse occurs mostly during the first 2 years after treatment, late relapse can also occur in 1.4–3.2% of patients [3]. Very late relapse (>5 years after diagnosis) is even rarer and occurs in 1% of patients. The most important observation from outcome studies of late relapse is that late relapse is usually chemoresistant and surgical resection of all sites of recurrence is essential for cure. In the Indiana University series, 59% of patients were treated with primary surgery for late relapsed GCT, of whom 92% had no evidence of disease at the last follow-up [4].
The development of a secondary SM in GCT is rare and remains poorly understood. The most common type of SM is sarcoma, which occurs in >50% of cases [5]. The next most common are various types of carcinoma [2]. SM occurs in 3–6% of primary testicular non-seminomatous GCTs (NSGCTs) and in 8% of post-chemotherapy RPLNDs; in over 20% of late-relapse cases [2]. The late-relapse SM from a stage I seminoma in case 2 is surprising because most SMs develop after systemic chemotherapy for disseminated NSGCT. There has been only one case report of SMs developed from pure seminoma [6]. In that case, the patient experienced a late relapse at nine years after he was cured from testicular seminoma clinical stage IIc by orchiectomy and cisplatin-based chemotherapy. Three hypotheses would explain the possible mechanisms of transformation of tumor from stage I pure seminoma to completely differentiated histology of SMs in our case 2. The first hypothesis assumes that there was a very small metastasis containing some foci of teratoma or even yolk sac tumor at first presentation, which escaped radiological detection due to their small size. The minuscule foci of teratoma persisted at the site of the metastasis and gave rise to the SMs at the time of clinical late relapse. The second hypothesis would suggest transformation and development of somatic malignant cells from seminoma cells at the site of the undetectable metastasis at first presentation although this pathway had not been conceived. The third hypothesis would suggest the presence of totipotent germ cells gave rise to differentiation into teratoma and SMs. However, no particular proof of any specific pathway can be given and the pathogenesis of SMs with seminoma in our case 2 remains indeterminate.
Although the optimal therapeutic strategy for SM has not been established because of the rarity of SM, GCT-oriented chemotherapy is not usually successful [2]. The rate of clinical CR with initial chemotherapy was reported to be ~13% for SM. Surgical resection can provide substantial benefits to patients if the tumor is localized and resectable. However, there have been a few reports of successful treatment of metastatic SMs with doxorubicin-based chemotherapy and a chemotherapy regimen with vincristine, dactinomycin and cyclophosphamide alternating with vincristine and irinotecan [7–9]. Chemotherapy directed against transformed component may achieve better results [8]. Tumor profiling by immunohistochemistry analysis may help to identify potential target and biomarkers and therapies associated with clinical benefit [9]. The role of radiation therapy for late relapsed tumor with SM remains poorly understood. There has been one report of metastatic late relapsed GCT successfully managed with radiation therapy [10]. Chemotherapy against transformed component and/or radiation therapy may have roles for unresectable tumor.
This case report described two cases of very late-relapse SM of GCT. Case 1 developed lung metastasis 13 years after cisplatin-based chemotherapy followed by RPLND for a stage IIA NSGCT. Case 2 developed a large pelvic tumor after 10 years of surveillance for stage I seminoma. Histopathologically, case 1 had SM consisting of adenocarcinoma, and case 2 had mixed SMs consisting of adenocarcinoma, rhabdomyosarcoma and glioblastoma. In both cases, we performed induction GCT-oriented chemotherapy followed by surgical resection. Because late-relapse tumors and SM are refractory to GCT-oriented chemotherapy [2], the combination of late-relapse GCT and SM is considered to be more refractory to chemotherapy. Indeed, the tumors increased in size after chemotherapy. Surgical resection played a major role in the treatment of both cases: case 1 experienced long-term surgical CR after surgery, and case 2 experienced normalized tumor markers, and no relapse for at least several months after surgery.
CONFLICT OF INTEREST STATEMENT
None declared.



