-
PDF
- Split View
-
Views
-
Cite
Cite
Erica C. Prochaska, Andrew P. Sciallis, Barbra S. Miller, Retroperitoneal calcifying fibrous tumor mimicking an adrenal tumor, Journal of Surgical Case Reports, Volume 2016, Issue 6, June 2016, rjw049, https://doi.org/10.1093/jscr/rjw049
Close - Share Icon Share
Abstract
Establishing the etiology of a retroperitoneal tumor may be difficult due to close proximity of multiple organs. Evaluation of retroperitoneal tumors often leads to surgery, many times to obtain a definitive diagnosis and rule out malignancy. Calcifying fibrous tumors (CFT) are very rare soft tissue tumors occurring most often in young patients. They are most often found arising in the thoracic cavity, mediastinum, abdominal cavity and extremities and usually have a benign clinical course. Macrocscopically, the tumors are well circumscribed and firm with a white-tan appearance. Histologically, CFT comprised a hypocellular proliferation of bland spindle cells, densely hyalinized collagen, chronic lymphoplasmacytic inflammation and dystrophic calcifications. Other considerations in the pathologic differential diagnosis include solitary fibrous tumor and inflammatory myofibroblastic tumor.
Introduction
Calcifying fibrous tumor (CFT) is a very rare, usually benign soft tissue tumor characterized by a proliferation of bland spindled mesenchymal cells with abundant collagen, background lymphoplasmacytic inflammation and variable amounts of stromal calcification. Affected patients tend to be young. Many cases are discovered incidentally. While CFT may recur, a benign clinical course is expected in 90%. Herein, we report the case of a patient with CFT arising within soft tissue densely adherent to the left adrenal gland.
Case Report
A 22-year-old man was evaluated and treated from age 10 for chronic epigastric pain. EGD and other diagnostic studies failed to elucidate a cause. Computed tomography (CT) imaging in 2003 revealed normal anatomy. Various medical management strategies failed to improve his symptoms. He underwent CT imaging which revealed a solid 6.2 × 5.9 × 4.8 cm well-circumscribed retroperitoneal mass thought to be emanating from the lateral limb of the left adrenal gland (Fig. 1a). The mass was abutting the posterior pancreatic tail and antero-superior aspect of the left kidney. The medical, family and social history was either unremarkable or noncontributory. Abdominal examination revealed no pain in the epigastric region and no palpable mass. Adrenal biochemical studies, including ACTH, cortisol, dehydroepiandrosterone sulfate, free testosterone, aldosterone, renin, plasma fractionated metanephrines, were within normal ranges. An A.M. cortisol was appropriately suppressed after administration of 1 mg dexamethasone. Serum tumor markers CA 19-9, CEA and chromogranin A were within normal ranges. Insulin, proinsulin, c-peptide and glucose levels were normal.
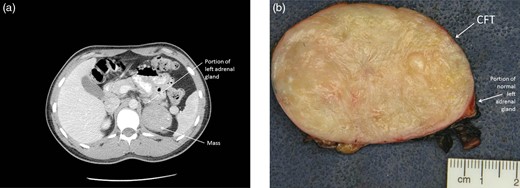
(a) CT scan of the abdomen showing a 6.2 × 5.9 × 4.8 cm retroperitoneal mass appearing to emanate from the lateral limb of the left adrenal gland. (b) Photograph of the gross specimen revealing a firm, white-tan and well-circumscribed tumor. A portion of normal adrenal tissue is visible.
Given the concern for an adrenocortical or other malignancy (pancreatic or soft tissue), an exploratory laparotomy via subcostal incision was performed. Findings revealed a firm, circumscribed mass emanating from the posterior aspect of the left adrenal gland. The mass did not involve any adjacent structures and there was no evidence of lymphadenopathy. A left adrenalectomy with en bloc resection of periadrenal soft tissue was performed without violation of the capsule. The patient had an uneventful postoperative course and was discharged from the hospital. The patient’s long-standing epigastric pain remains unchanged.
Macroscopically, the mass was firm, white-tan and well circumscribed (Fig. 1b). Histologically, the mass comprised a hypocellular proliferation of bland spindle cells, densely hyalinized collagen, chronic lymphoplasmacytic inflammation and dystrophic calcifications (Fig. 2). While there was no infiltration into the adrenal gland, there was early entrapment of small vessels. Most of the spindle cells that appeared morphologically fibroblastic were normochromatic, and had a low mitotic rate (0–1 per 10 high-powered fields). Multinucleation and cellular atypia were both absent. The growth pattern was variable with haphazard and storiform foci. The inflammatory component was chiefly composed of small lymphocytes and plasma cells. There were scattered, non-psammomatous dystrophic calcifications.
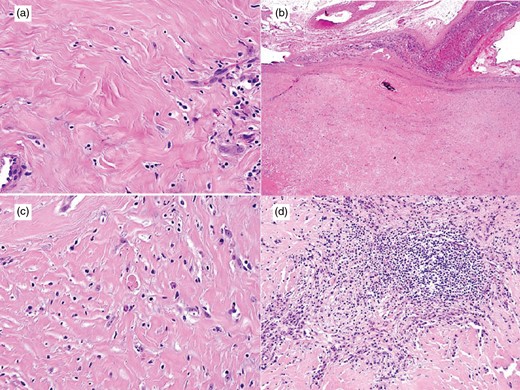
(a,b) CFT demonstrating hypocellular proliferation of bland spindle cells, densely hyalinized collagen, chronic lymphoplasmacytic inflammation and dystrophic calcifications (H&E; x400 and x20). (c,d) Inflammatory component of CFT, comprised small lymphocytes and plasma cells, the latter are occasionally binucleate or have Russell bodies (H&E; x200 and x400).
Immunophenotypically, the spindle cells were negative for smooth muscle actin, desmin, ALK, S100, CD34 and CD117. There were rare CD117-positive mast cells. The plasma cells were polytypic without restricted kappa or lambda light chain expression, and the majority expressed immunoglobulin G (IgG) with a subset positive for IgG4. In three x400 plasma cell-rich fields (‘hotspots’), the average number of IgG-positive cells was 183 and the average number of IgG4-positive cells was 11 (ratio = 16) (Fig. 3).
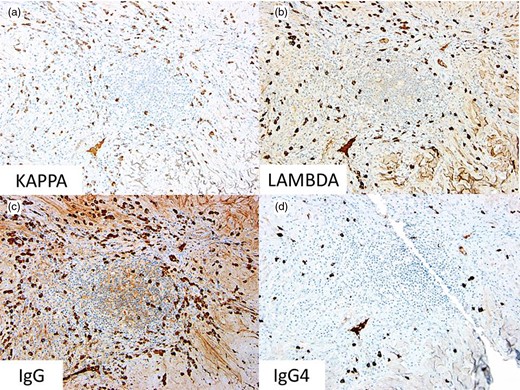
Immunohistochemical stains for (a) kappa light chain expression (x200), (b) lambda light chain expression (x200), (c) IgG (x200) and (d) IgG4 (x200).
Discussion
CFT, initially described by Rosenthal and Abdul-Karim [1] in 1988 (‘childhood fibrous pseudotumor with psammoma bodies’), was further characterized by Fetsch et al. [2] in 1993 (‘calcifying fibrous pseudotumor’), and then reported as a series of 15 cases by Nascimento et al. [3] in 2002. CFTs can arise in a variety of locations, but are more commonly described arising in or from the pleura, thoracic cavity, extremities, mediastinum and abdominal cavity [3, 4]. To date, there have only been four previously reported cases of adrenal CFT [5–8]. These lesions were previously called calcifying fibrous pseudotumor. However, due to a local recurrence rate of ~10%, these lesions were renamed CFTs in the current World Health Organization classification [9].
This case is similar to the one reported by Lau and Weiss [5], although in that particular case, the mass nearly replaced the entire adrenal gland, whereas in this current case, the adrenal gland was spared. Closer scrutiny of these previous cases suggests that the tumors were perhaps adjacent to rather than arising from the adrenal gland as in this case [5–8]. The gross description, histology, immunophenotype and polytypic nature of the plasma cells are identical.
Radiologic characterization of these masses is not well described other than being solid tumors that are usually well circumscribed. Calcifications are varied in distribution. Magnetic resonance imaging findings described in a case report revealed signal iso-intense to skeletal muscle on T1 weighted images and low signal intensity on T2 weighted images. Other types of benign fibrous tumors reveal intense peripheral enhancement after gadolinium injection; however, the CFT showed only mild peripheral enhancement [10]. 18F-fluorodeoxyglucose positron emission tomography–CT findings are usually indicative of a benign tumor.
Histologically, CFTs are composed of a hypocellular proliferation of non-atypical spindle cells, dense collagen, lymphoplasmacytic inflammation and stromal calcifications. In the majority of cases, the spindle cells are negative for actins, S100, desmin and ALK [3]. CD34 expression is more variable and can be focal or diffuse.
Similar to CFT, inflammatory myofibroblastic tumor (IMT) contains a proliferation of spindle cells accompanied by inflammation-containing plasma cells. Dystrophic calcifications are rare. Spindle cells of IMT are usually positive for muscle specific actin, SMA and desmin, but staining can be focal. Of note, 25–40% of extrapulmonary IMTs recur, but metastasis is rare (<2% of cases). The histological similarities between CFT and IMT have raised the question as to whether CFTs are a sclerosing end stage of IMTs. Both lesions consist of spindle cells, collagen deposition and lymphocytic infiltrates. Despite similarities, the majority of CFTs are immunophenotypically distinct from IMT.
In conclusion, CFT is an uncommon soft tissue lesion of uncertain etiology low on the list of differential diagnoses often occurring in younger patients in a variety of locations and usually associated with a clinically benign course. The features of CFT can overlap with other spindle cell neoplasms such as IMT.
Conflict of interest statement
None declared.
References
- adrenal glands
- inflammation
- cancer
- adrenal mass
- adrenal gland neoplasms
- collagen
- differential diagnosis
- limb
- neoplasms, fibrous tissue
- retroperitoneal neoplasms
- retroperitoneal space
- soft tissue neoplasms
- surgical procedures, operative
- diagnosis
- mediastinum
- neoplasms
- surgery specialty
- pseudotumor, inflammatory
- solitary fibrous tumor
- thoracic cavity
- causality
- calcifying fibrous pseudotumor
- calcification
- Abdominal cavity



