-
PDF
- Split View
-
Views
-
Cite
Cite
Thais O. Polanco, Sara Alothman, Hector Depaz, Alexius Ramcharan, A rare case of listeriosis, acute cholecystitis and multiple myeloma, Journal of Surgical Case Reports, Volume 2016, Issue 5, May 2016, rjw080, https://doi.org/10.1093/jscr/rjw080
Close - Share Icon Share
Abstract
Listeria monocytogenes (LM) is an aerobic, motile, intracellular gram-positive bacterium. Most invasive systemic infections caused by LM are commonly seen in patients at both extremes of age, during pregnancy or in immunocompromised hosts. Common clinical manifestations of LM infection in immunocompromised adults are bacteremia, infections of central nervous system, such as meningitis, and self-limiting febrile gastroenteritis. Focal infections of listeria are rare, especially cholecystitis, with only few cases reported in the last 33 years. A 62-year-old man presented with multiple myeloma, cholecystitis and LM bacteremia. Due to prompt surgical treatment and antibiotics (amoxicillin plus clavulanic acid and gentamycin), this high-risk patient recovered without any complications.
Introduction
Listeria monocytogenes (LM) is an aerobic, motile, intracellular gram-positive bacterium. The primary habitat of Listeria is soil and decaying vegetable matter [1]. It has been isolated in numerous human food products, water and sewage systems. LM is known to colonize the gut and present asymptomatically in feces in 1–12% of healthy individuals [1, 2]. In the USA, 1651 cases of listeriosis were identified between 2009 and 2011 in the Foodborne Diseases Active Surveillance Network. Of these cases, 58% occurred in patients of 65 years or older, 29% in non-pregnant patients younger than 65 years and 14% in pregnant women [1].
Most systemic infections caused by LM are commonly seen in patients at extremes of age, during pregnancy or in immunocompromised hosts [1, 3]. Glucocorticoid therapy, immunosuppressive treatment and conditions such as hematologic malignancies are the most important predisposing factors to listeriosis in non-pregnant populations. Listeriosis has various presentations, depending on the patient population and their associated risk factors. Common clinical manifestations of LM infection in immunocompromised adults are bacteremia, infections of central nervous system, such as meningitis, and self-limiting febrile gastroenteritis. Focal infections of listeria are rare, especially cholecystitis, with only few cases reported in the last 33 years [2, 4–6]. We report a case of acute cholecystitis in a 62-year-old man with listeriosis and multiple myeloma.
Case Report
A 62-year-old man presented to the emergency department with fever, abdominal pain, watery diarrhea and general malaise for 2 weeks. He reported five copious, watery, blood-streaked bowel movements per day associated with epigastric pain. The pain was non-radiating, stabbing in nature, with a severity score of 6/10. Bowel movements partially relieved the pain, but consumption of solid foods aggravated it. He had nausea but no vomiting. Four days prior to presentation, he had fever and chills. His medical history was remarkable for multiple myeloma, gastroesophageal reflux disease, cytomegalovirus (CMV), epstein-Barr virus, Hepatitis B and urinary incontinence. Medications included lenalidomide 25 mg, aspirin 81 mg, dexamethasone 4 mg, omeprazole 20 mg, tenofovir 300 mg, valacylovir 500 mg and oxybutynin 5 mg daily. He had no toxic habits.
On examination, he had a temperature of 99.7°F; pulse 86 beats/minute; respirations 20/minute and blood pressure 136/60 mmHg. There was no icterus. No abdominal scars present. Bowel sounds were normal. The abdomen was soft, distended and tender in the right upper quadrant and epigastrium with no guarding or rebound tenderness. The rest of the examination was normal.
Hemoglobin 11.9 g/dl, white blood cell count 4.6/mm3 and platelet counts 73 cells/nl. The morphology of the white blood cells showed few smudge cells and Dohle bodies. Serum electrolyte studies were normal except for potassium 2.9 mmol/l and creatinine level 1.4 mg/dl. The aspartate aminotransferase and alanine aminotransferase level were normal. Alkaline phosphatase level was 49 U/l, total bilirubin level 1.2 mg/dl and lactate level 3.5 mmol/l. Amylase level was 65 units, and lipase was 347 units.
Electrocardiogram showed sinus tachycardia with frequent premature ventricular complexes. Abdominal and pelvic computed tomographic (CT) scan revealed a distended gallbladder with calculi in the fundus, gallbladder wall thickening and pericholecystic fluid [Fig. 1]. Diffuse osteopenia and multiple punched-out lytic lesions in the axial skeleton consistent with multiple myeloma were reported on the CT scan.
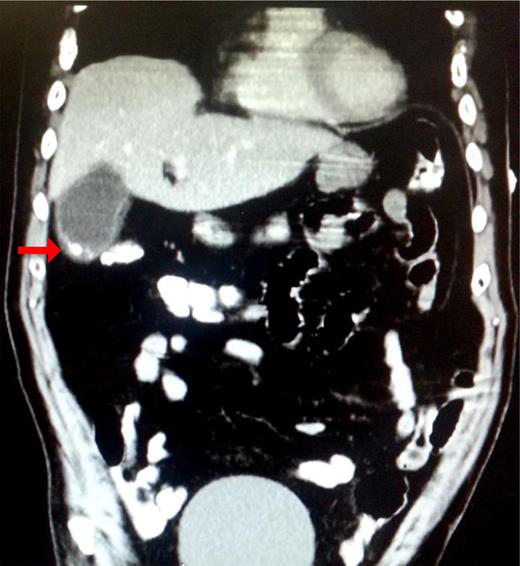
Mural calcification and/or stone in the fundus of the gallbladder (red arrow).
The patient was resuscitated with intravenous infusion of normal saline, potassium chloride and calcium gluconate. He received 500 mg of metronidazole and 3.375 g of piperacillin sodium–tazobactam. He underwent laparoscopic cholecystectomy without complication. Histopathological examination of the gallbladder report confirmed the diagnosis of acute cholecystitis (at least Grade 2, according to the Tokyo guidelines) [7]. There were no features consistent with CMV cholecystitis noted in the pathology report. Blood cultures were positive for LM on postoperative Day 1. Subsequent viral studies revealed positive cytomegalovirus antibody immunoglobulin M positive. He received intravenous ampicillin/sulbactam (2 g, every 4 hours) and gentamicin (1 mg/kg, every 8 hours) for 2 weeks, in addition to his home medications.
He was extubated on Day 1 after his operation and was alert and oriented. Once patient successfully recovered from cholecystitis and listeriosis, he remained hospitalized for workup for leukopenia and thrombocytopenia. He was discharged on postoperative Day 19.
Discussion
Microbiological examination of the bile, gallbladder and blood cultures is not routinely performed in patients with cholecystitis. LM is a rare cause of biliary tract infection and acute cholecystitis. LM is able to survive and replicate in extreme environmental conditions. The ability to induce biliary tract infection is a general property of LM. After ingestion, the bacterium uses its virulence factors such as, bile salt hydrolase and bile exclusion system, to infect the gastrointestinal tract [8, 9]. Contang et al. studied the process of infection of LM in a murine gallbladder. It showed that Listeria was able to replicate intra- and extracellularly in the murine gallbladder [10]. This indicates that the LM can induce overt cholecystitis and biliary tract infection. Lecuit et al. presented 20 culture-proven listeria biliary tract infection cases from 1996 to 2013 [2]. The cases consisted of 17 cholecystitis, 2 cholangitis and 1 biliary cyst infection. Blood cultures were positive in 60% of the patients. Gallbladder histology lesions were analyzed in three patients and showed acute, chronic or necrotic infections. Other acute cholecystitis with listeriosis case reports were noted in literature over the last 33 years [3–5].
Even though LM is infrequent, it is considered to be a cause of cholecystitis in high-risk patient populations. It is a possibility that due to the lack of blood cultures and microbiological examinations of the gallbladder, it continues to remain undetected in clinical practice. LM can cause a life-threatening systemic illness in humans—with high mortality rate of 20–30% in high-risk individuals [6]. This immunocompromised patient with multiple myeloma and cholecystitis serves as a reminder of the possible lethal association of acute cholecystitis and listeriosis if clinically missed. To decrease the morbidity and mortality in these patient groups, it is essential that the patient be provided with prompt surgical treatment and appropriate antibiotics. For future cases, it would be ideal to preform gallbladder biopsy to reliably culture the pathogenic organism.
Conflict of interest statement
None declared.
References
- amoxicillin
- antibiotics
- pregnancy
- cholecystitis, acute
- cholecystitis
- gentamicin sulfate (usp)
- sepsis
- meningitis
- bacteremia
- central nervous system infection
- listeria monocytogenes
- fever
- focal infections
- adult
- clavulanic acid
- gastroenteritis
- gentamicins
- gram-positive bacteria
- immunocompromised host
- listeria
- listeriosis
- signs and symptoms
- surgical procedures, operative
- multiple myeloma
- infections



