-
PDF
- Split View
-
Views
-
Cite
Cite
Simon Goldie, Jack Sandeman, Richard Cole, Simon Dennis, Ian Swain, Electrical stimulation treatment for facial palsy after revision pleomorphic adenoma surgery, Journal of Surgical Case Reports, Volume 2016, Issue 4, April 2016, rjw057, https://doi.org/10.1093/jscr/rjw057
Close - Share Icon Share
Abstract
Surgery for pleomorphic adenoma recurrence presents a significant risk of facial nerve damage that can result in facial weakness effecting patients’ ability to communicate, mental health and self-image. We report two case studies that had marked facial weakness after resection of recurrent pleomorphic adenoma and their progress with electrical stimulation. Subjects received electrical stimulation twice daily for 24 weeks during which photographs of expressions, facial measurements and Sunnybrook scores were recorded. Both subjects recovered good facial function demonstrating Sunnybrook scores of 54 and 64 that improved to 88 and 96, respectively. Neither subjects demonstrated adverse effects of treatment. We conclude that electrical stimulation is a safe treatment and may improve facial palsy in patients after resection of recurrent pleomorphic adenoma. Larger studies would be difficult to pursue due to the low incidence of cases.
Introduction
Eighty-five percent of salivary gland tumors of the parotid gland are pleomorphic adenomas [1]. Surgical excision is the recognized treatment as around 16.1% demonstrate histological features of malignancy [2].
Surgical management includes total parotidectomy, superficial parotidectomy and extracapsular dissection. All demonstrate low recurrence risk of between 5 and 9.7%, but carry significant risks of facial nerve damage [1, 2].
Effects of facial nerve damage can include facial asymmetry, synkinesis and Frey's syndrome that can impact an individual's self-image, mental health and ability to communicate [1, 3]. These risks are further increased in revision surgery. In total, 95% of patients will demonstrate a House–Brackmann score more than Grade II and 11.3% will demonstrate a score of Grade III or more 1 year after surgery [2]. This may be a consequence of greater infiltration of the facial nerve or increased surgical difficulty due to disturbed anatomy [2, 4].
Electrical stimulation (ES) involves placing surface electrodes over the affected nerve to stimulate and recover its function. The stimulator generates an action potential in both nerves and muscle tissues that has been demonstrated in animal models to promote their structural recovery [5]. We report our promising findings of two cases treated with ES for facial weakness after revision surgery for pleomorphic adenoma.
Case Reports
Two subjects were referred to the National Clinical Functional Electrical Stimulation Centre with significant facial palsy after revision pleomorphic adenoma surgery:
Subject A was a 35-year-old woman who had significant left facial weakness after surgery for her third recurrence of pleomorphic adenoma. She had previously been treated with enucleation at age 15, superficial left parotidectomy at age 30 and local resection using an intraoral approach at age 34. Three months after surgery, her House–Brackmann score was Grade IV with a Sunnybrook score of 54 due to resting asymmetry and weakness particularly effecting the left side of the mouth.
Subject B was a 25-year-old man who had significant facial asymmetry after a right superficial parotidectomy for pleomorphic adenoma with repair of a communicating branch between the zygomatic and buccal facial nerve branches. Previous treatments included removal of a presumed right sebaceous cyst at age 15 (histologically demonstrated pleomorphic adenoma) with facial nerve branch repair.
One month after surgery, his House–Brackmann score was Grade VI with a Sunnybrook score of 64. His face was symmetrical at rest; however, he had significant reduction of right-side mouth movement on smiling.
Stimulation was provided by a two-channel microprocessor-controlled stimulator (microstim2v2(MS2v2) [Odstock Medical Limited]) producing 300 μs balanced monophasic pulses at 40 pps with an output of up to 120 mA. This was applied to both patients’ affected sides overlying the buccal branch of the facial nerve using PALS Plus electrodes (Axelgaard).
The unaffected side was stimulated initially to find motor points and estimate thresholds for contraction of the contralateral side. Electrodes were placed as close as possible together to ensure that the current path was superficial.
The subjects were shown how to apply the electrodes and use the stimulator. They completed two sessions per day each lasting 15 minutes initially. This was increased by 5 minutes per session per week until a target of 30 minutes twice a day was reached. Subjects were reviewed at set up and 2, 6, 10, 18 and 26 weeks after.
At reviews, digital photographs, facial measurements, House–Brackmann and Sunnybrook scores were taken. Facial measurements were mapped during expressions using an engineer's vernier caliper (Table 1). Comparison of measurements between the affected and unaffected sides of the face indicated the severity of asymmetry.
Measurements depicting distances (in millimeters) between corner of the mouth and various markers when performing expressions.
| Distance (mm) . | Subject A . | Subject B . | ||||||
|---|---|---|---|---|---|---|---|---|
| Pre-ES . | Post-ES . | Pre-ES . | Post-ES . | |||||
| R side . | L side . | R side . | L side . | R side . | L side . | R side . | L side . | |
| Lateral canthus—corner of mouth when smiling | 61 | 74 | 67 | 67 | 71 | 67 | 60 | 62 |
| Lateral canthus—corner of mouth when kissing | 74 | 76 | 79 | 79 | 80 | 82 | 74 | 75 |
| Corner of mouth—ear lobe when smiling | 83 | 93 | 85 | 85 | 84 | 79 | 84 | 84 |
| Distance (mm) . | Subject A . | Subject B . | ||||||
|---|---|---|---|---|---|---|---|---|
| Pre-ES . | Post-ES . | Pre-ES . | Post-ES . | |||||
| R side . | L side . | R side . | L side . | R side . | L side . | R side . | L side . | |
| Lateral canthus—corner of mouth when smiling | 61 | 74 | 67 | 67 | 71 | 67 | 60 | 62 |
| Lateral canthus—corner of mouth when kissing | 74 | 76 | 79 | 79 | 80 | 82 | 74 | 75 |
| Corner of mouth—ear lobe when smiling | 83 | 93 | 85 | 85 | 84 | 79 | 84 | 84 |
R = Right; L = Left.
Measurements depicting distances (in millimeters) between corner of the mouth and various markers when performing expressions.
| Distance (mm) . | Subject A . | Subject B . | ||||||
|---|---|---|---|---|---|---|---|---|
| Pre-ES . | Post-ES . | Pre-ES . | Post-ES . | |||||
| R side . | L side . | R side . | L side . | R side . | L side . | R side . | L side . | |
| Lateral canthus—corner of mouth when smiling | 61 | 74 | 67 | 67 | 71 | 67 | 60 | 62 |
| Lateral canthus—corner of mouth when kissing | 74 | 76 | 79 | 79 | 80 | 82 | 74 | 75 |
| Corner of mouth—ear lobe when smiling | 83 | 93 | 85 | 85 | 84 | 79 | 84 | 84 |
| Distance (mm) . | Subject A . | Subject B . | ||||||
|---|---|---|---|---|---|---|---|---|
| Pre-ES . | Post-ES . | Pre-ES . | Post-ES . | |||||
| R side . | L side . | R side . | L side . | R side . | L side . | R side . | L side . | |
| Lateral canthus—corner of mouth when smiling | 61 | 74 | 67 | 67 | 71 | 67 | 60 | 62 |
| Lateral canthus—corner of mouth when kissing | 74 | 76 | 79 | 79 | 80 | 82 | 74 | 75 |
| Corner of mouth—ear lobe when smiling | 83 | 93 | 85 | 85 | 84 | 79 | 84 | 84 |
R = Right; L = Left.
Subjects’ images of facial symmetry pre- and post-treatment are presented in Fig. 1. They initially demonstrated significant asymmetry when smiling that markedly improved at treatment conclusion.
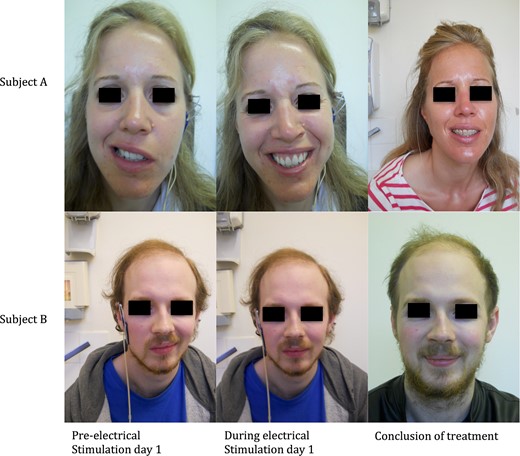
Facial symmetry of subjects performing a smile before, during and after treatment with ES.
Facial measurements initially demonstrated a large difference in the distance between the unaffected and affected side in both patients. Both subjects showed a marked improvement in symmetry when smiling after treatment (Table 1).
Subject A had an initial Sunnybrook score of 54 that improved to 88. Her House–Brackmann score improved from Grade IV to II. Her resting symmetry demonstrated drooping of the cheek and mouth on the affected side that resolved after treatment. Initially there was only slight movement of mouth opening, snarling and lip puckering on the affected side that improved to almost complete movement. Subject B had an initial Sunnybrook score of 64 that improved to 96. His House–Brackmann score improved from Grade IV to II. At the initiation of the study, he had no resting asymmetry. Prior to stimulation, his ability to open his mouth, snarl and pucker his lip only showed slight movement, but recovered to complete movement in all domains except lip pucker (Table 2).
| Sunnybrook . | Subject A . | Subject B . | ||
|---|---|---|---|---|
| Score criteria . | Pre-ES . | Post-ES . | Pre-ES . | Post-ES . |
| Symmetry of voluntary movement | ||||
| Forehead wrinkle | 5 | 5 | 5 | 5 |
| Gentle eye closure | 4 | 5 | 5 | 5 |
| Smile | 2 | 4 | 2 | 5 |
| Snarl | 2 | 4 | 2 | 5 |
| Lip pucker | 3 | 4 | 2 | 4 |
| Total | 16 | 22 | 16 | 24 |
| Total × 4 | 64 | 88 | 64 | 96 |
| Resting symmetry | ||||
| Eye shape | 0 | 0 | 0 | 0 |
| Nasolabial fold | 1 | 0 | 0 | 0 |
| Mouth | 1 | 0 | 0 | 0 |
| Total | 2 | 0 | 0 | 0 |
| Total × 5 | 10 | 0 | 0 | 0 |
| Synkinesis with voluntary movement | ||||
| Forehead wrinkle | 0 | 0 | 0 | 0 |
| Gentle eye closure | 0 | 0 | 0 | 0 |
| Smile | 0 | 0 | 0 | 0 |
| Snarl | 0 | 0 | 0 | 0 |
| Pucker | 0 | 0 | 0 | 0 |
| Total | 0 | 0 | 0 | 0 |
| Total composite score | 54 | 88 | 64 | 96 |
| Sunnybrook . | Subject A . | Subject B . | ||
|---|---|---|---|---|
| Score criteria . | Pre-ES . | Post-ES . | Pre-ES . | Post-ES . |
| Symmetry of voluntary movement | ||||
| Forehead wrinkle | 5 | 5 | 5 | 5 |
| Gentle eye closure | 4 | 5 | 5 | 5 |
| Smile | 2 | 4 | 2 | 5 |
| Snarl | 2 | 4 | 2 | 5 |
| Lip pucker | 3 | 4 | 2 | 4 |
| Total | 16 | 22 | 16 | 24 |
| Total × 4 | 64 | 88 | 64 | 96 |
| Resting symmetry | ||||
| Eye shape | 0 | 0 | 0 | 0 |
| Nasolabial fold | 1 | 0 | 0 | 0 |
| Mouth | 1 | 0 | 0 | 0 |
| Total | 2 | 0 | 0 | 0 |
| Total × 5 | 10 | 0 | 0 | 0 |
| Synkinesis with voluntary movement | ||||
| Forehead wrinkle | 0 | 0 | 0 | 0 |
| Gentle eye closure | 0 | 0 | 0 | 0 |
| Smile | 0 | 0 | 0 | 0 |
| Snarl | 0 | 0 | 0 | 0 |
| Pucker | 0 | 0 | 0 | 0 |
| Total | 0 | 0 | 0 | 0 |
| Total composite score | 54 | 88 | 64 | 96 |
Composite score = symmetry of voluntary movement × 4 − ([resting facial symmetry × 5] + synkinesis with voluntary movement). Maximum score of 100.
| Sunnybrook . | Subject A . | Subject B . | ||
|---|---|---|---|---|
| Score criteria . | Pre-ES . | Post-ES . | Pre-ES . | Post-ES . |
| Symmetry of voluntary movement | ||||
| Forehead wrinkle | 5 | 5 | 5 | 5 |
| Gentle eye closure | 4 | 5 | 5 | 5 |
| Smile | 2 | 4 | 2 | 5 |
| Snarl | 2 | 4 | 2 | 5 |
| Lip pucker | 3 | 4 | 2 | 4 |
| Total | 16 | 22 | 16 | 24 |
| Total × 4 | 64 | 88 | 64 | 96 |
| Resting symmetry | ||||
| Eye shape | 0 | 0 | 0 | 0 |
| Nasolabial fold | 1 | 0 | 0 | 0 |
| Mouth | 1 | 0 | 0 | 0 |
| Total | 2 | 0 | 0 | 0 |
| Total × 5 | 10 | 0 | 0 | 0 |
| Synkinesis with voluntary movement | ||||
| Forehead wrinkle | 0 | 0 | 0 | 0 |
| Gentle eye closure | 0 | 0 | 0 | 0 |
| Smile | 0 | 0 | 0 | 0 |
| Snarl | 0 | 0 | 0 | 0 |
| Pucker | 0 | 0 | 0 | 0 |
| Total | 0 | 0 | 0 | 0 |
| Total composite score | 54 | 88 | 64 | 96 |
| Sunnybrook . | Subject A . | Subject B . | ||
|---|---|---|---|---|
| Score criteria . | Pre-ES . | Post-ES . | Pre-ES . | Post-ES . |
| Symmetry of voluntary movement | ||||
| Forehead wrinkle | 5 | 5 | 5 | 5 |
| Gentle eye closure | 4 | 5 | 5 | 5 |
| Smile | 2 | 4 | 2 | 5 |
| Snarl | 2 | 4 | 2 | 5 |
| Lip pucker | 3 | 4 | 2 | 4 |
| Total | 16 | 22 | 16 | 24 |
| Total × 4 | 64 | 88 | 64 | 96 |
| Resting symmetry | ||||
| Eye shape | 0 | 0 | 0 | 0 |
| Nasolabial fold | 1 | 0 | 0 | 0 |
| Mouth | 1 | 0 | 0 | 0 |
| Total | 2 | 0 | 0 | 0 |
| Total × 5 | 10 | 0 | 0 | 0 |
| Synkinesis with voluntary movement | ||||
| Forehead wrinkle | 0 | 0 | 0 | 0 |
| Gentle eye closure | 0 | 0 | 0 | 0 |
| Smile | 0 | 0 | 0 | 0 |
| Snarl | 0 | 0 | 0 | 0 |
| Pucker | 0 | 0 | 0 | 0 |
| Total | 0 | 0 | 0 | 0 |
| Total composite score | 54 | 88 | 64 | 96 |
Composite score = symmetry of voluntary movement × 4 − ([resting facial symmetry × 5] + synkinesis with voluntary movement). Maximum score of 100.
Neither patient suffered from Frey's syndrome or synkinesis, and both have sustained their facial symmetry for 3 and 15 months after treatment.
Discussion
Interest in ES began in the late 1950s focusing on facial paresis after Bell's palsy.
Alkaram et al. [6] noted no significant changes in House–Brackmann scores of those treated for early Bell's palsy with ES compared with no treatment. In contrast, Tucany et al. [7] noted the addition of ES to physical therapy and corticosteroids significantly improved House–Brackmann scores in subjects with acute Bell's palsy.
Targan et al. [8] assessed those with stable chronic facial palsies due to Bell's palsy (lasting on average 3.7 years) and surgical sacrifice (lasting on average 7.2 years) noting significant improvement in House–Brackmann scores after ES treatment.
Unfortunately, there are many differences between studies such as stimulator settings and duration of treatment, which make comparison difficult. To our knowledge, no studies have evaluated ES in patients after revision pleomorphic adenoma surgery.
In our case reports, both subjects had unchanged severe facial palsy since revision pleomorphic adenoma excision for 1 and 3 months postoperatively. During treatment with ES, their facial palsy drastically improved from Sunnybrook scores of 54–88 and 64–96. Neither subject suffered complications. These findings suggest that ES may benefit patients with facial palsy after revision pleomorphic adenoma surgery. Both subjects felt their facial palsy was stable prior to treatment; however, it is possible that spontaneous recovery may have occurred. Unfortunately, larger studies would be difficult to pursue due to a low incidence of cases.
Conflict of Interest Statement
I.S. has a financial relationship with Odstock Medical Ltd (OML), the company that manufactures and distributes the MS2v2 stimulator used for treatment. He was seconded to OML by Salisbury NHS Trust for 30% wte. OML is majorly owned by Salisbury NHS Foundation Trust and pays the Trust a license fee for use of the patents. No royalties are paid to the named patent authors. The other authors have nothing to disclose.



