-
PDF
- Split View
-
Views
-
Cite
Cite
Peter Waweru, Hardeep Gill, Chris Abeid, Protracted refractory pain post-TEVAR: post-implantation syndrome?, Journal of Surgical Case Reports, Volume 2016, Issue 10, October 2016, rjw173, https://doi.org/10.1093/jscr/rjw173
Close - Share Icon Share
Abstract
Aortic dissection is a life-threatening condition and has one of the highest mortality rates of cardiovascular diseases. It remains a devastating disease; with multiple unanswered questions concerning treatment modalities. The role of thoracic endovascular aortic repair (TEVAR) in these patients; especially those with uncomplicated acute aortic Type B dissections (AAD-B) is especially controversial although it has been shown to have better long-term outcomes compared to medical therapy alone. For those who have TEVAR, up to 60% may develop an acute, transient systemic inflammatory response syndrome that remains vaguely defined. The role of local inflammation in this post-implantation syndrome (PIS) has not been highlighted. We present a case of a 57-year-old male patient with an uncomplicated AAD-B who developed an ‘atypical’ PIS post-TEVAR with severe refractory abdominal pains; leukocytosis and raised C-reactive protein. The role of local inflammation in PIS is highlighted.
Introduction
Aortic dissection is a life-threatening condition affecting up to 30 people per million each year [1]. It remains a devastating disease with one of the highest mortality rates of cardiovascular diseases and multiple unanswered questions regarding treatment [2]. Endovascular repair of uncomplicated acute aortic Type B dissections (AAD-B) is especially controversial [3] and its associated post-implantation syndrome (PIS) vague [4]. We present a case of a 57-year-old man with an uncomplicated AAD-B managed with TEVAR; and the subsequent refractory pains, only later managed with steroids as atypical PIS. Although most definitions do not include local features of inflammation (including pain) as part of PIS; its place in systemic inflammatory response syndrome (SIRS) and PIS is highlighted.
Case Report
A 57-year-old man of African descent known to have hypertension; presented with acute onset, progressively worsening chest pain radiating to the back (severity >8; numeric pain rating scale). He had no other comorbidities, was a non-smoker and did not volunteer any history of alcohol use. He was on metoprolol, irbesartan and hydrochlorothiazide for his hypertension; which he admitted to using only when he felt unwell.
On examination, he had a blood pressure of 172/119 (right arm) and 137/92 (left arm). The rest of the cardiac examination was unremarkable. He had a full complement of pulses with no bruits or murmurs. High-sensitivity Troponin T was elevated (19.29 ng/ml), but not rising. An electrocardiogram (ECG) and a transthoracic 2D-echocardiogram showed left ventricular hypertrophy and no wall motion abnormalities. A computed tomography aortogram (CTA) was done showing an aortic dissection involving the descending aorta extending to the left common iliac, with iliac, superior mesenteric and both renal arteries arising from true lumen (Fig. 1a). He was admitted to the intensive care unit and started on morphine and labetalol infusion at 2 mg/h.
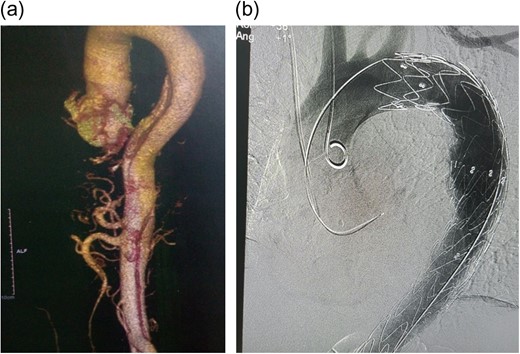
(a) CT Aortogram showing the dissection, 3D reconstruction; (b) immediate post-TEVAR showing contrast only in true lumen.
The patient was selected for TEVAR according to Cooper's [5] proposed algorithm and the decision reinforced by the history of non-compliance to medical therapy. A Valiant Captiva (Medtronic) 38 × 200 mm stent was deployed through the right femoral artery into the aorta, landing proximally just distal to the left subclavian artery and distally just distal to the celiac artery with an overlap of 110 mm and effective occlusion of the false lumen (Fig. 1b). Of note, 12 h post-TEVAR however; the patient started complaining of colicky, non-specific abdominal pains; associated with a leukocytosis, decreased platelet counts, the absence of fever and rising C-reactive protein (CRP) (Fig. 2). He was managed conservatively with non-steroidal anti-inflammatory agents (NSAIDs), antispasmodics and proton pump inhibitors (PPIs). Five days later, pains persisted, increasing in severity; with minimal relief with NSAIDs and antispasmodics. He also had thrombocytopenia, leukocytosis and elevated CRP. The abdomen was soft, surgical sites clean and bowel sounds present. An array of tests were done including procalcitonin (normal); blood cultures (negative); liver function tests (normal), serum amylase/lipase (normal); abdominal ultrasound (minimal gall bladder sludge); repeat ECGs (no changes); oesophagoscopy (normal) and magnetic resonance imaging of the thoracic and lumbar spine (normal). A repeat CTA showed the graft in place without endoleaks. In total, 16 days post procedure; he was started on intravenous steroids (for possible PIS) with dramatic clinical improvement and subsequently discharged home after 48 h in a stable condition.
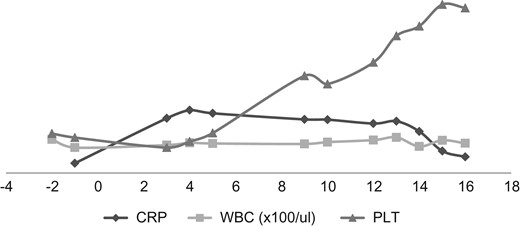
Chart showing laboratory markers of PIS in our patient. Note the initial attenuation of CRP, with normalization of WBC/PLT on fifth day post-TEVAR and the drastic drop in CRP/PLT with initiation of steroids on Day 14. WBC, white blood cell counts; PLT, platelets.
Discussion
Described over two centuries ago; aortic dissections result from an intimal tear that leads blood into a false lumen between the intimal and medial layers of the aortic wall [5]. Management remains complex and mortality high [1]. As classified by Daily et al. [6] in 1970 at Stanford; Type A dissections involve the ascending aorta and Type B involve only the descending aorta. The latter account for over a third of aortic dissections [1, 5]; a majority hypertension-related and are uncomplicated—so assigned by the absence of malperfusion, hypotension, refractory hypertension or findings suggestive of impending rupture [2].
Once a diagnosis of an uncomplicated AAD-B is established, a majority of patients are started on medical therapy; and this has been widely accepted as the standard treatment [2, 3]. This entails intensive anti-impulse therapy; the cornerstone of which is reduction of pressure on the aortic wall and subsequent decreased propagation of the false lumen. While a majority of patients so treated are discharged with no complications; the long-term outcomes are perturbing with high mortality and complication rates [7]. Subsequently, questions abound about the possibility of multimodal therapy with adjunctive TEVAR in a bid to improve the outcomes [5]. This stent–graft placement occludes the primary intimal tear promoting false lumen thrombosis and subsequent aortic remodeling [3]; with resultant reduction of disease progression, late adverse events and aorta specific mortality [7, 8]. Despite this, however, the use of TEVAR in uncomplicated AAD-B remains controversial; and is not without risks, one of which is PIS.
First described in 1999, PIS, is a vaguely defined SIRS following endovascular repair that presents with a noninfectious fever, leukocytosis (>12 000/μl), elevated CRP (>10 mg/L) and coagulation disturbances including thrombocytopenia in the context of negative blood culture results; and occurs in up to 60% patients [4]. Though the exact pathophysiology remains an enigma, it is believed to result from complex endothelial-stent fabric interactions where tissue injury activates complement and induces production of various pro-inflammatory mediators including tumor necrosis factor-α, interleukin (IL)-1, IL-6; with local and systemic effects [4]. While the systemic effects are well appreciated, and define PIS; there is hardly any mention of its local effects, including local pain.
In Kenya, compliance to antihypertensives has been shown to be low [9], and thus an added benefit with TEVAR in management of this case postulated. While the patient developed evident SIRS with tachycardia, leukocytosis, thrombocytopenia and high serum CRP post-TEVAR; it is his refractory pain that was worrying. Evaluation for the etiology of this, from endoleaks to cord ischemia yielded no positive result and thus the eventual management as an atypically presenting PIS, refractory to NSAIDs. Although PIS is a largely systemic inflammatory response; perigraft inflammation has been shown in experimental studies [10] and thus features of local inflammation (like pain in our case) should be borne in mind to avoid delayed treatment with associated lengthy hospital stays.
Authors’ contributions
H.G. was the primary doctor in the care of this patient and was involved in providing data needed (including images) in preparation of this report. He was also very resourceful in literature review on the same. C.A. was the physician in care of the patient, in view of his hypertension. He was also very resourceful in literature review especially on hypertension locally and internationally; and in the preparation of the manuscript, including revising it critically for important intellectual content. P.W. was the lead author with significant contribution in case report framework, literature review, draft revision and final submission. All authors approved the final draft before submission.
Acknowledgements
Dr Nabeel Farouq and Dr Emmanuel Oundo for their role in this patient's care and acquisition of images used in this case report, respectively.
Consent for publication
Patient consent was obtained (available for review if needed) for publication of this case report.
Conflict of interest statement
None declared.
Funding
No funding was obtained for this case report.



