-
PDF
- Split View
-
Views
-
Cite
Cite
Elizabeth W. Tan, Mark D. Smith, A rare occurrence of hepatic portal venous gas in a patient with chemotherapy-induced enterocolitis: the rise of benign aetiologies, Journal of Surgical Case Reports, Volume 2015, Issue 9, September 2015, rjv114, https://doi.org/10.1093/jscr/rjv114
Close - Share Icon Share
Abstract
Hepatic portal venous gas (HPVG) is often viewed as an ominous imaging finding with a poor prognosis and a high mortality rate. We recently encountered a case of HPVG in a patient with advanced metastatic prostate cancer previously treated with chemotherapy and radiotherapy. A laparotomy was performed, which was negative. Although HPVG secondary to chemotherapy is extremely rare, we as clinicians need to consider this aetiology and other benign aetiologies. With the increased rate of benign aetiologies and their successful conservative management, the role of emergency laparotomies needs to be re-considered.
INTRODUCTION
Hepatic portal venous gas (HPVG) is a rare radiological sign often associated with intestinal ischaemia requiring emergency laparotomy. This case report will discuss a 77-year-old Caucasian man with gastrointestinal symptoms, neutropenic sepsis and HPVG as seen on competent tomography. A review of the literature will also be conducted with regard to the pathophysiology, conditions associated with HPVG and the role of conservative management in non-ischaemic pathologies.
CASE REPORT
A 77-year-old male was transferred from a rural hospital; he initially presented with symptoms of left iliac fossa pain, diarrhoea, nausea, vomiting and fevers for 3 days duration. He has a past history of advanced prostate cancer with bony metastasis, previous chemotherapy and radiotherapy and type two diabetes mellitus requiring insulin. Regular medications are abiraterone, prednisolone, flutamide, oxycodone and targin. General examination revealed sinus tachycardia and an elevated temperature. On abdominal examination, there was diffuse tenderness worse in the left iliac fossa, but soft with positive bowel sounds.
Blood investigations revealed a low haemoglobin of 104 g/l (115–165 g/l), low white cell count 1.56 × 109/l (4.0–10.0 × 109/l) with normal coagulation, liver function, lipase and lactate parameters. Competent tomography (CT) abdomen scan identified grossly thickened small bowel with a large amount of portal venous gas in liver (Figs 1 and 2). Like us, the radiologist was concerned these features were highly suggestive of small bowel ischaemia.
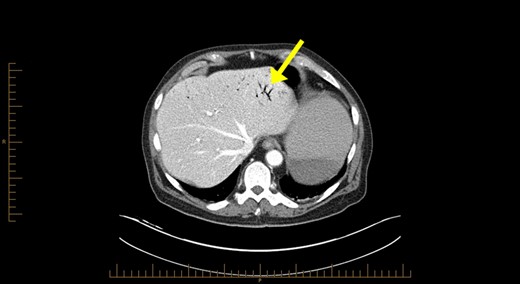
Computerized tomography abdominal axial section showing air within the hepatic portal veins (yellow arrows).
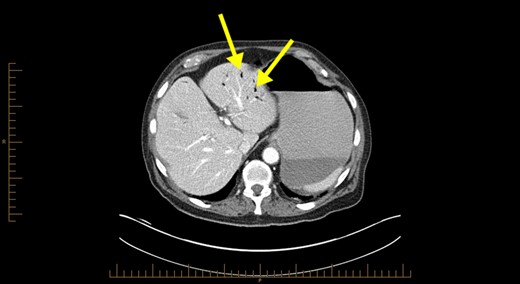
Computerized tomography abdominal axial section showing air within the hepatic portal veins (yellow arrows).
However, with no peritoneal signs, he was treated conservatively and admitted to Intensive Care for further management of neutropenic sepsis. He was fluid resuscitated, commenced on intravenous tazocin, granulocyte-colony stimulating factor (GCSF) and nasogastric decompression. No source of infection was found on blood, stool and urine cultures.
With a rising C-reactive protein of 560 mg/l (1–10 mg/l) and his clinical condition, an exploratory laparotomy was performed. Intra-operative findings were moderate bowel oedema, but no bowel or mesenteric necrosis. His abdomen was closed after division of adhesions of the jejunum and ileum. The patient was apprised of the likely diagnosis of chemotherapy/radiotherapy-related severe enterocolitis with adhesions. Twenty-seven days post-admission and a conservatively managed complication of small bowel obstruction, he was transferred back to the rural hospital to be closer to his family.
DISCUSSION
In Libman's and Kinoshita's studies of 64 cases and 182 cases, respectively [1, 2], HPVG was an ominous imaging finding necessitating urgent laparotomy. It had a 75% mortality rate in 43% of Kinoshita's study population that was associated with intestinal ischaemia [2]. Progression in imaging technology from plain abdominal radiography to CT scans has since altered the significance of this radiological finding, leading to a rise in clinically benign aetiologies. These include inflammatory bowel disease, abdominal trauma, post-chemotherapy and a rare complication of endoscopic procedures [3]. In ischaemic pathologies, the sensitive and timely detection have allowed prompt treatment, significantly reducing overall mortality rates to 29–39% [4, 5].
The pathophysiology of HPVG is unclear, but two hypotheses in the literature exist. The first states that factors such as mucosal barrier damage, bowel distension, increased intraluminal pressure and bacterial fermentation of carbohydrates in sepsis result in gas production [5]. The second theory is that gas-forming organisms present in the intestinal wall or venous system causes HPVG [5]. As seen in Fig. 1, HPVG is characteristically distributed as peripheral radiolucencies extending to within 2 cm of the liver capsule.
With the increased incidence of benign pathologies, many authors in the literature now question the need for emergency surgery [3–5]. In the case of our elderly frail patient with a positive radiological sign of HPVG but no acute abdominal signs, the initial trial of conservative treatment was deemed appropriate. However, with minimal improvement and deterioration, an explorative laparotomy was warranted to definitively exclude the possibility of intra-abdominal sepsis with intestinal necrosis.
With the negative laparotomy, we conclude that the HPVG was likely triggered by chemotherapy-induced enterocolitis, causing small bowel dilatation and oedema with resultant mucosal damage. It is difficult to distinguish which subcategory of chemotherapy-induced enterocolitis it is. It could be neutropenic enterocolitis with his classic symptoms of fevers and neutropenia; however the pathology did not occur 10-14 days after chemotherapy initiation and had no caecal involvement [6]. A relatively newer type of enterocolitis due to taxane agents such as docetaxel- used in the treatment of metastatic prostate cancer [6] also fits our patient's presentation however no ischaemic bowel was found. Also, his adhesions were likely due to radiotherapy.
In other studies, the possibility that certain chemotherapy drugs resulting in intestinal mucosa damage leading to HPVG could not be excluded [7–9]. Kung et al. [9] reports HPVG in a patient taking irinotecan and cisplatin who had normal bowel findings on subsequent laparotomy. A common side effect of irinotecan is gastrointestinal toxicity, and Kung et al. concludes that when combined with cisplatin, it likely led to mucosal ulceration, bowel distension and anaerobic bacteria gas production [9]. Sakamoto et al. [10] further describes a patient with non-small cell lung cancer who developed HPVG on a chemotherapy regimen of carboplatin and paclitaxel. He concludes the effects of chemotherapy agents and the increased intraluminal pressure due to severe constipation from opioid analgesics resulted in HPVG [10]. Thus with our patient, it is plausible that his previous chemotherapy treatment and long-term opioid analgesics could have caused his HPVG.
In conclusion, the presence of HPVG alone is not a surgical indication, as it is no longer only associated with fatal aetiologies with a poor prognosis. However, more research needs to be conducted into the mechanism behind HPVG and its association with chemotherapy agents. To reduce the rate of negative laparotomies and post-operative complications, we suggest that in cases of HPVG where ischaemic abdominal pathologies have been excluded; a conservative approach should be trialed.
CONFLICT OF INTEREST STATEMENT
None declared.



