-
PDF
- Split View
-
Views
-
Cite
Cite
Michael Kueht, Prakash Masand, Abbas Rana, Ronald Cotton, John Goss, Concurrent hepatic hemangioma and solitary fibrous tumor: diagnosis and management, Journal of Surgical Case Reports, Volume 2015, Issue 7, July 2015, rjv089, https://doi.org/10.1093/jscr/rjv089
Close - Share Icon Share
Abstract
Hepatic solitary fibrous tumor (HSFT) is a very rare benign liver tumor without well-defined findings on imaging. Even with multiphase advanced contrast-enhanced liver imaging, a definitive preoperative diagnosis is impossible. The diagnostic process can be further complicated when there are two concurrent lesions with different radiologic appearances. Here, we compare the findings of a commonly encountered liver lesion, hepatic hemangioma, with those of an exceedingly rare lesion, HSFT.
INTRODUCTION
Hepatic solitary fibrous tumor (HSFT) is a very rare lesion arising from mesothelial cells within the liver. Due to a usually indolent presentation and nonspecific radiographic findings, it cannot be definitively diagnosed preoperatively, and hepatic incidentaloma has been increasingly discovered [1]. The decision to intervene on a hepatic incidentaloma is made only after a thorough diagnostic investigation has been undertaken, including deliberate imaging. The clinical picture can be further complicated when there are two concurrent lesions with different radiologic appearances. Here, we present a case of a patient in whom the investigation of an incidentally discovered hepatic hemangioma led to the discovery of a second difficult-to-diagnose lesion, eventually found to be a primary HSFT.
CASE REPORT
A 40-year-old male underwent computed tomography (CT) scan of the chest for atypical chest pain, which revealed an indeterminate 3-cm lesion in the right liver. He subsequently underwent triple-phase CT, which revealed two separate lesions: a 4.3 × 5.3-cm discrete mass in the left liver (Segment 2) and a 2.3 × 3.7-cm lobular, partially exophytic hypodense lesion in the right liver (Segment 6). On the delayed phase, the periphery of the right posterior hepatic mass was isodense with the rest of the liver parenchyma, but the central portion remained hypodense. Based on these findings, the right-sided lesion was felt to be a hemangioma, but the findings were still inconclusive regarding the left-sided lesion. Magnetic resonance imaging (MRI) was performed with intravenous gadoxetic acid, a hepatocyte-specific contrast agent. The lesion in Segment 2 was hypointense on precontrast T1, hyperintense on T2-weighted images, and showed avid enhancement on the arterial and portal venous phases. There was a delayed washout on the hepatographic phase. Although nonspecific, this was felt to be most consistent with an adenoma. The right-sided lesion showed postcontrast gradual enhancement with centripetal fill-in, reinforcing the diagnosis of hemangioma (Figs 1 and 2).
We decided to observe the right-sided lesion, confidently diagnosed as an uncomplicated hemangioma. His liver function and platelet counts were normal, and the chest pain was resolved. However, as the left-sided lesion was felt to possibly be a large hepatic adenoma, we advised surgery. After cardiopulmonary clearance, the patient underwent left lateral segmentectomy utilizing intraoperative ultrasound. We found a firm, tan-white mass on which a frozen section was performed. This preliminary read was consistent with a spindle cell tumor without malignant features, and so the resection was limited to the left lateral segment.
The patient's postoperative course was fortunately uneventful, and he was discharged on the fifth postoperative day.
Histological analysis of the lesion revealed a 4.7 × 4.0 × 4.0-cm mass surrounded by normal liver parenchyma. Immunohistochemical staining revealed the tumor cells to be positive for CD 34, BCL-2, CD 99 and vimentin, and negative for pan cytokeratin, cam 5.2, S 100, CD 31, CD 117 and Dog-1. They were focally positive for desmin and caldesmon, muscle-specific actin and heavy-chain myosin. Again, malignant features were not identified (no areas of hypercellularity, cytologic atypia, necrosis, increased mitoses or infiltrative margins) [2].
The combination of gross appearance and histologic features was most compatible with a benign primary HSFT, and no further treatment was administered (Fig. 3).
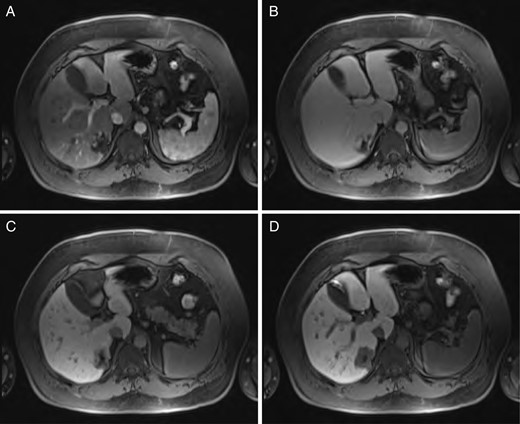
Sequential MRI imaging of hepatic hemangioma. Note delayed centripetal filling: (A) T1 arterial phase, (B) T1 60-s delay phase, (C) T1 postcontrast phase and (D) T1 hepatographic phase.
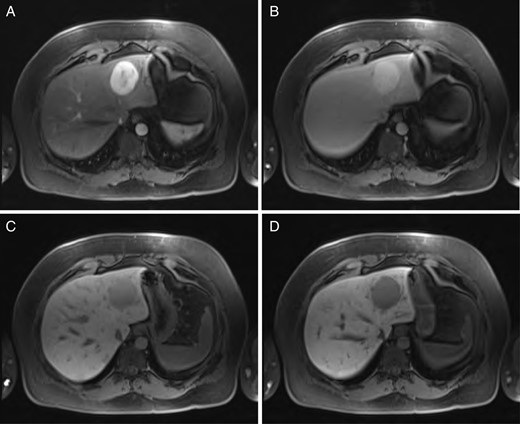
Sequential MRI imaging of HSFT: (A) T1 arterial phase, (B) T1 60-s delay phase, (C) T1 postcontrast phase and (D) T1 hepatographic phase.
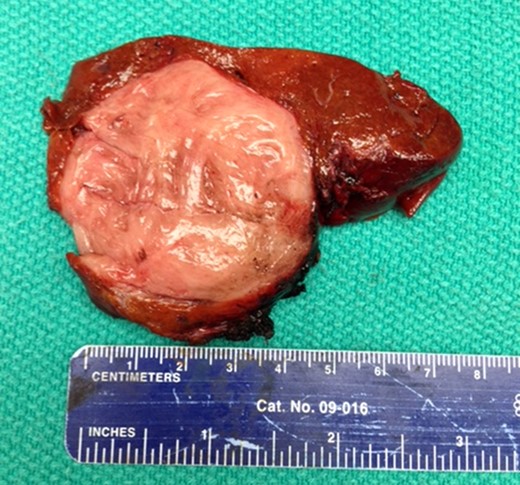
Gross appearance of HSFT. Note whorled appearance and normal surrounding parenchyma.
DISCUSSION
Hepatic incidentalomas in adults are usually benign, and in most cases, modern radiological techniques reliably demonstrate a diagnosis [1, 3]. Nonetheless, we are sometimes unable to establish a definitive diagnosis with noninvasive means. In fact, the inability to rule out malignancy is a common indication for resection [4].
Liver biopsy is contraindicated in most primary hepatic tumors due to the risk of bleeding, and therefore, the diagnosis relies heavily on radiographic findings. For instance, hepatocellular carcinoma has pathognomonic radiographic features that are enough to make the diagnosis and begin treatment without a tissue sample, especially in the setting of an elevated alpha-feto protein level [5]. Other benign liver lesions also have characteristic radiographic findings that allow patients to avoid unnecessary intervention.
Hepatic hemangioma is a common hepatic incidentaloma that has characteristic features on both CT and MRI. Triple-phase imaging will reveal a hypoattenuating lesion on the early phase, while the delayed phases show peripheral isoattenuation (with the rest of the liver parenchyma) with a persistently hypoattenuating central portion that gradually fills [6]. This is due to the centripetally oriented vascular drainage of the lesion, flowing from the periphery toward the center.
Indications for resection of hemangiomas are few and are mainly related to size and symptomatology. An adage at our institution is to ‘never, never, never’ operate on hepatic hemangiomas unless they are ‘very, very, very’ implying that although these lesions are highly vascular, complications are usually limited to those that are exceedingly large [6].
On the other hand, hepatic adenoma has less specific imaging findings and a higher risk of complications. It is accepted that all hepatic adenomas should be considered for resection due to the malignant potential and risk of rupture, although surgery is not commonly undertaken for lesions <5 cm in diameter [3]. The use of multiphase MRI with gadoxetic acid contrast has the benefit of an additional phase specific to hepatocytes, as it is processed in the same manner as bile salts [7].
A review of HSFT by Liu and colleagues found 10% to have malignant features [8]. In all cases, the diagnosis was made after resection.
In summary, the decision to operate on an incidentally discovered liver mass in a patient without significant risk factors for malignancy is best accomplished by a multidisciplinary approach. Targeted imaging studies with a priori goals, whether or not tissue biopsy is necessary or feasible, and the surgeon's confidence in his or her ability to safely remove the lesion are some of the aspects of care that need coordination, usually in a multidisciplinary face-to-face discussion. Evaluation for malignancy takes priority, and when this cannot be ruled out, surgery is favored. In our patient, there were two incidentally discovered lesions, each requiring different treatments. As of now, with primary HSFT, the sole modality of definitive diagnosis is histological analysis after surgical resection.
CONFLICT OF INTEREST STATEMENT
There are no previous publications or presentations that might be regarded as redundant publication of the same or very similar work. There are no previously published abstracts for meeting presentations that contain partial or similar material to this paper. There is no permission required from publication sources. None of the authors possess possible conflicts of interest and/or commercial involvement.
ACKNOWLEDGEMENT
The authors thank Eric Silberfien, MD, for his contribution of surgical adages.



