-
PDF
- Split View
-
Views
-
Cite
Cite
Pietro G. di Summa, Adrien Yvon, Lorenz Larcher, Wassim Raffoul, Nathalie Koch, Propionibacterium avidum infection following breast reduction: high morbidity from a low-virulence pathogen, Journal of Surgical Case Reports, Volume 2015, Issue 2, February 2015, rjv002, https://doi.org/10.1093/jscr/rjv002
Close - Share Icon Share
Abstract
Propionibacterium avidum is a common inhabitant of sebaceous glands, traditionally considered to be of low virulence and generally found on implanted foreign material. We report a rare case of P. avidum breast abscess, causing severe morbidity following breast reduction surgery. A 36-year-old woman presented with a non-painful wound discharge 3 weeks postoperatively, and was treated conservatively. She was readmitted 7 weeks postoperatively with a red and tender breast. A purulent discharging abscess was drained under ultrasound guidance. A 2-week intravenous course of amoxicillin–clavulanic acid, followed by oral replacement for a month resulted effective. Serial ultrasound imaging was useful in treatment decision-making. The infective potential of P. avidum may be underappreciated. Proximity of sutures to the axilla, tobacco smoking and the potential for resorbable sutures to host bacteria may predispose to infection, and should raise the clinician's awareness.
INTRODUCTION
Reduction mammoplasty is a common surgical procedure for symptomatic breast hypertrophy. Postoperative complications include haematoma, infection, fat necrosis and delayed wound healing. Among infection agents, Staphylococcus aureus is the most common primary infective organism. Propionibacterium avidum [1–4] (P. avidum), a common inhabitant of sebaceous glands, is traditionally considered to be of low virulence. We report one of a few cases of P. avidum in breast tissue, and to our knowledge, the third described following breast reduction. We recommend an effective treatment for similar cases, drawing from the conclusions of previous literature and our own experience.
CASE REPORT
A 36-year-old Caucasian heavy smoker (1 pack/day), with no other comorbidities, presented with bilateral symptomatic breast hypertrophy. Reduction mammoplasty was performed following Thorek's technique [5]. Standard disinfection was performed with antiseptic povidone–iodine and 600 mg of intravenous (IV) clindamycin was delivered at anaesthesia induction. More than 1.1 kg of tissue was retrieved from each breast. Three weeks postoperatively, wound dehiscence associated with discharge was noted on the inferior border of the right breast. Neither redness nor heat was detected locally. The patient was apyrexial with no inflammatory syndrome (WBC count 11 g/l and C-reactive protein 6 mg/l). A smear of the discharge with liponecrosis showed low quantities of P. avidum. No antibiotic therapy was introduced at this moment and dressings with argentic sulfadiazine were prescribed every 48 h (Ialugen-plus, IBSA, Switzerland). Seven weeks postoperatively, a new red and tender induration in between the inferior quadrants of the right breast was noted. Interestingly, the patient remained apyrexial with no inflammatory syndrome. A breast ultrasound (US) showed a 4 × 4 × 15 cm encapsulated collection (Fig. 1). The abscess was drained and bacteriological examination of the purulent fluid revealed a larger quantity of P. avidum. Amoxicillin–clavulanic acid three times/day IV was started as empirical treatment for soft tissue infection. During the IV antibiotic treatment, no differences were seen in blood test parameters; however, a clinical improvement was evident with the discharge steadily decreasing. A control breast US on Day 5 post IV treatment showed remission of the abscess (Fig. 2). Results of antibiogram analysis confirmed the bacteria presence and its sensitiveness to amoxicillin–clavulanic acid. IV antibiotherapy was continued cessation of wound dehiscence (2 weeks), and then switched to oral amoxicillin for 1 month (750 mg three times a day). A breast US 1 month later showed abscess resolution and antibiotic therapy was discontinued. On physical examination, the infection had healed, and no surgical reintervention was needed (Fig. 3).
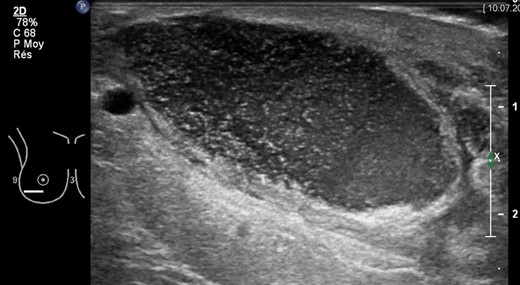
Right breast ultrasonography (US) showing encapsulated purulent collection (4 × 4 × 15 cm) in correspondence of the lower lateral quadrant, 7 weeks after breast reduction surgery. Abscess drainage was performed under US guidance.
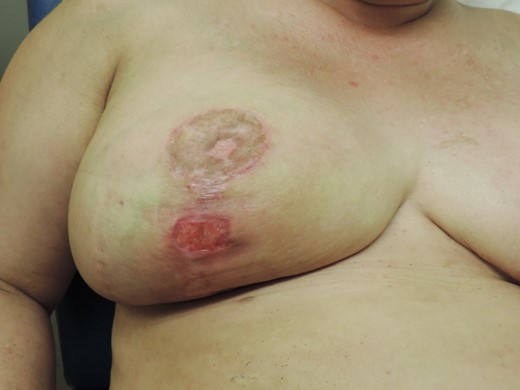
Clinical status after US-guided drainage and 2 weeks IV antibiotic therapy.
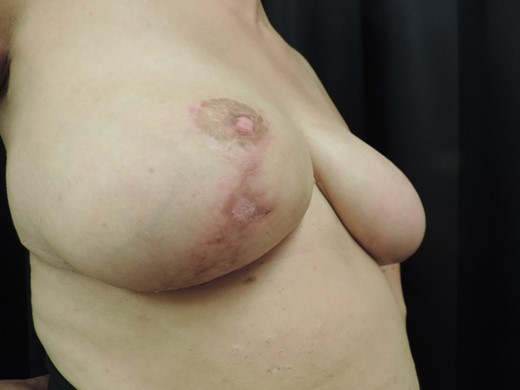
Complete wound healing after 6 weeks of antibiotic treatment and local dressings.
DISCUSSION
Propionibacteria are anaerobic, gram-positive bacilli and are a genus of Coryneform bacteria. The incubation period of Propionibacteria infections can last from a few days to several months [6]. Our case is consistent with previous reports that have described the insidious nature of infections with P. avidum following surgery [4]. This organism is a usual inhabitant of sebaceous glands, and hair follicles in the groin, perineal and axillary regions. The slow growth of P. avidum and its commensal role as a skin organism means the infections can more easily be overlooked. Typically, P. avidum is encountered in the presence of implanted foreign material, which is an ideal terrain for bacterial adherence and biofilm formation [6]. Other described predisposing factors contributing to P. avidum infections include immunosuppression, trauma, malignancy, diabetes and obstruction of sinuses or ducts [6]. Tobacco smoking in our case is likely to have increased the pathogen's morbidity postoperatively, severely affecting wound healing and supporting liponecrosis. Even if we could consider breast reduction surgery as a ‘clean procedure’, scar proximity to the axilla needs to be included among contamination risks. In the few described cases of P. avidum infections after breast reduction surgery, several antibiotic treatments have been used (Table 1). Our antibiogram was consistent with previous reports showing P. avidum to be sensitive to most antibiotics including penicillins, but not to metronidazole [4, 6]. Briefly, the initial choice was to administer beta-lactamase-resistant penicillins IV, followed by an oral replacement for up to 1 month. For possible penicillin allergies [2], an IV carbapenem (meropenem) was used prior to an oral fluoroquinolone (moxifloaxacin). Propionibacterium avidum is likely to be found in the presence of implanted foreign materials. It produces an exopolysaccharide-like meshwork that has been hypothesized to play a role in biofilm formation [7], leading to persistent infections that cannot be cleared by the immune system. While no prosthetic material is present in reduction mammoplasty, sutures may host bacteria during their resorption phase, which can last over 70 days. Moreover, the contamination can proceed through the filaments and cause inflammation in the surrounding tissue [8]. In our case, braided resorbable sutures (Vycril™, Ethicon, Johnson & Johnson, USA) were used for deep layers, followed by resorbable monofilaments for the dermis and skin (Monosyn®, B. Braun Medical, Germany) [9, 10]. There is still debate whether braided or monofilament resorbable sutures should be used in contaminated surgical fields [9], with some human and in vivo studies showing greater infection rates in polyfilament than in monofilament sutures, although other studies suggest no difference [8, 10].
Antibiotic treatments for P. avidum infection following breast reduction surgery in the literature
| Surgery . | Treatment . | Time to complete healing . | Penicillin allergy . | Source . |
|---|---|---|---|---|
| Bilateral breast reduction–unilateral infection | IV AC for 2 weeks + 4 weeks oral AC | 6 weeks | Recalled possible allergy | Reported case |
| Bilateral breast reduction and infection | IV flucloxacillin and benzyl penicillin for 5 days + 4 weeks oral AC | 5 weeks | No | Panagea et al. [1] |
| Bilateral breast reduction and infection | IV meropenem + 4 weeks oral moxifloxacin + 4 weeks oral AC | 12 weeks | Recalled possible allergy | Levin et al. [2] |
| Unilateral mastectomy + TRAM flap reconstruction | IV flucloxacillin + penicillin | n/a | No | Werno et al. [4] |
| Surgery . | Treatment . | Time to complete healing . | Penicillin allergy . | Source . |
|---|---|---|---|---|
| Bilateral breast reduction–unilateral infection | IV AC for 2 weeks + 4 weeks oral AC | 6 weeks | Recalled possible allergy | Reported case |
| Bilateral breast reduction and infection | IV flucloxacillin and benzyl penicillin for 5 days + 4 weeks oral AC | 5 weeks | No | Panagea et al. [1] |
| Bilateral breast reduction and infection | IV meropenem + 4 weeks oral moxifloxacin + 4 weeks oral AC | 12 weeks | Recalled possible allergy | Levin et al. [2] |
| Unilateral mastectomy + TRAM flap reconstruction | IV flucloxacillin + penicillin | n/a | No | Werno et al. [4] |
AC, amoxicillin–clavulanic acid; IV, intravenous; TRAM, transverse rectus abdomini muscle.
Antibiotic treatments for P. avidum infection following breast reduction surgery in the literature
| Surgery . | Treatment . | Time to complete healing . | Penicillin allergy . | Source . |
|---|---|---|---|---|
| Bilateral breast reduction–unilateral infection | IV AC for 2 weeks + 4 weeks oral AC | 6 weeks | Recalled possible allergy | Reported case |
| Bilateral breast reduction and infection | IV flucloxacillin and benzyl penicillin for 5 days + 4 weeks oral AC | 5 weeks | No | Panagea et al. [1] |
| Bilateral breast reduction and infection | IV meropenem + 4 weeks oral moxifloxacin + 4 weeks oral AC | 12 weeks | Recalled possible allergy | Levin et al. [2] |
| Unilateral mastectomy + TRAM flap reconstruction | IV flucloxacillin + penicillin | n/a | No | Werno et al. [4] |
| Surgery . | Treatment . | Time to complete healing . | Penicillin allergy . | Source . |
|---|---|---|---|---|
| Bilateral breast reduction–unilateral infection | IV AC for 2 weeks + 4 weeks oral AC | 6 weeks | Recalled possible allergy | Reported case |
| Bilateral breast reduction and infection | IV flucloxacillin and benzyl penicillin for 5 days + 4 weeks oral AC | 5 weeks | No | Panagea et al. [1] |
| Bilateral breast reduction and infection | IV meropenem + 4 weeks oral moxifloxacin + 4 weeks oral AC | 12 weeks | Recalled possible allergy | Levin et al. [2] |
| Unilateral mastectomy + TRAM flap reconstruction | IV flucloxacillin + penicillin | n/a | No | Werno et al. [4] |
AC, amoxicillin–clavulanic acid; IV, intravenous; TRAM, transverse rectus abdomini muscle.
Our case provides evidence that P. avidum infections are not exclusive to hardware material or contaminated prosthesis, but can be encountered in breast reduction surgery, suggesting the potential biofilm formation on sutures. Propionibacterium avidum gives less striking clinical features when compared with other more common pathogens, and may rest underappreciated. Despite the low virulence of the pathogen, it can finally lead to severe morbidity. In breast reduction with long inframammary scars, and in the presence of predisposing factors such as smoking, diabetes or immunosuppression, P. avidum contamination may not remain silent. In such cases, the clinician's suspicion should be raised. According to our experience and given the previously reported cases, we suggest an initial IV treatment with amoxicillin–clavulanic acid until the cessation of wound discharge, followed by a 4-week oral course (or until complete wound healing). An US should be considered as a non-invasive and practical way to follow clinical evolution during antibiotic treatment and on an outpatient basis.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- propionibacterium
- smoking
- ultrasonography
- breast abscess
- abscess
- amoxicillin-potassium clavulanate combination
- axilla
- decision making
- foreign bodies
- pain
- sebaceous glands
- surgical procedures, operative
- sutures
- tobacco
- infections
- bacteria
- breast
- morbidity
- virulence
- pathogenic organism
- reduction mammaplasty
- ultrasonic guidance procedure
- wound discharge
- host (organism)



