-
PDF
- Split View
-
Views
-
Cite
Cite
Ahmed M. Al Maksoud, Adel K. Barsoum, Mohammed M. Moneer, Langer's arch: a rare anomaly affects axillary lymphadenectomy, Journal of Surgical Case Reports, Volume 2015, Issue 12, December 2015, rjv159, https://doi.org/10.1093/jscr/rjv159
Close - Share Icon Share
Abstract
Langer's arch is the best-known anatomic variant of definite surgical implication in the region of the axilla. This rare anomaly is a muscular slip extending from the latissimus dorsi (LD) muscle to the tendons, muscles or fasciae around the superior part of the humerus. In this report, we present a rare case of left axillary arch. During modified radical mastectomy for breast cancer, we encountered an abnormal muscle slip crossing the axilla from the LD muscle to the posterior surface of the pectoralis major muscle anterior to the neurovascular structures. Preoperative knowledge is essential to identify such unusual anomaly and avoid potential complications both intra- and postoperatively.
INTRODUCTION
Knowledge of the anatomical variations of the axillary region has become more relevant with increasing surgeries of this region for breast cancer, reconstruction procedures and axillary by-pass [1].
Lager's arch or the axillary arch (AA) is the best-known variant structure in the axilla. It is a muscular or fibromuscular slip of varying dimensions, extending from the latissimus dorsi (LD) muscle about the middle of the posterior axillary fold, and crosses over the neurovascular structures, to join the under surface of the tendon of the pectoralis major, the coracobrachialis, or the fascia over the bicepsbrachii [1, 2].
CASE REPORT
A 60-year-old female presented to our breast clinic with a left breast lump sized 3 × 2.5 cm incidentally felt since 3 months. It was gradually increasing in size and was not associated with any other breast complaints. She had relatively small-sized breasts. On triple assessment, she was evaluated with mammography that suggested a BIRADS 3 lesion at 12 O′clock position. Fine needle aspiration cytology showed ductal carcinoma of the breast.
Subsequently, her metastatic work-up included abdominal pelvic ultrasound, chest computed tomography and bone scan. There was no evidence of metastatic disease.
She was planned for a modified radical mastectomy. Intra-operatively, during the axillary lymph node dissection, we encountered an unusual muscle slip crossing the axilla from the LD muscle to the posterior surface of the pectoralis major muscle anterior to the axillary vein. All neurovascular structures and lymphoid tissue were lying posterior to this abnormal muscle (Fig. 1).
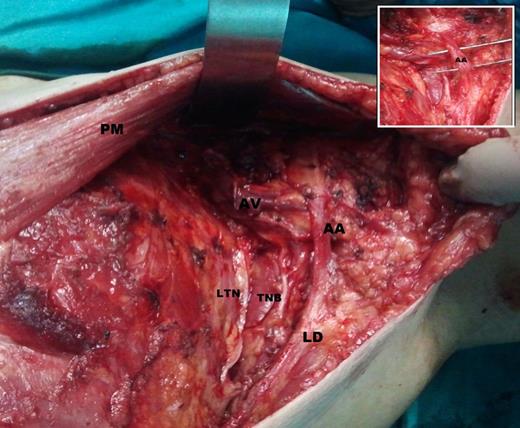
AA muscle extending from LD muscle to under surface of pectoralis major tendon in the left axilla crossing over axillary neurovascular structures (in view). AA, axillary arch; PM, pectoralis major; LD, latissimus dorsi muscle; AV, axillary vein; TNB, thoracodorsal neurovascular bundle; LTN, long thoracic nerve.
The lymph nodes lateral and beneath the arch were successfully dissected, and the arch itself was left undisturbed. The procedure was uneventful, and the patient had a good post-operative recovery.
Pathological assessment revealed invasive duct carcinoma Grade 2. Tests for oestrogen, progesterone and HER 2 receptors were negative. All 20 axillary lymph nodes were free from metastatic deposits.
On follow-up, 21 months after surgery, the patient was alive and free of known disease.
DISCUSSION
The axillary ach was first identified by Ramsay in 1795. However, it was Karl Langer in 1846 who gave a more accurate description of this variant, so thereafter, it was named after him [2–4].
Throughout the literature, several terms have been used to describe the muscular variant running from the LD muscle towards the pectoralis major muscle: ‘AA′, ‘Langer's AA’, ‘AA muscle’, ‘axillopectoral muscle’ and also their translations in different languages [2]. In the following text, we choose to use the name ‘axillary arch’ (AA).
The anatomical description of the AA is variable among authors. According to Testu's classification (1884), the complete AA extended between the LD and the tendon of the pectoralis major near its insertion on the humerus; the incomplete one extended from the LD to the axillary fascia, biceps brachii muscle, coracobrachialis muscle, the distal end of the bicipital groove and the inferior edge of pectoralis minor muscle or coracoid process [2]. The case we encountered was of unilateral complete AA.
According to many anatomic texts, the AA is present in 7% of population. However, it has been recognized in only 0.25% during axillary surgical procedures [1, 2, 4, 5].
The difference in surgical and anatomical incidence reflects a failure of reporting or identification during surgery. Also, this discrepancy may be attributed to the specific aim of cadaveric studies to identify such anatomic anomalies [5].
Clinically, AA can be palpable during physical examination as an axillary mass. This should be kept in mind as it can be confused with axillary lymphadenopathy or soft tissue tumour [1, 3].
AA can act as entrapment site for the neurovascular bundle during some arm movements causing circulatory deficiency, chronic pain and paraesthesia. Simple division of the arch is curative in such cases [2, 5].
Jelev, through his extensive work, introduced a new definition of ‘clinical AA’ as a variant muscular structure in the axilla that is a possible entrapment site for the nerves and vessels. He further classified clinical arches into superficial and deep. In clinical practice, the existence of a superficial or deep AA could be suspected according to the vessels or nerves mostly affected [2].
Magnetic resonance imaging is a helpful tool to diagnose AA. It can positively correlate its presence with neurovascular entrapment symptoms. Also, it can assess its anatomic relations [1, 5].
The surgical significance of the AA is 2-fold: (i) it may hide some axillary nodes, and (ii) it may mislead the dissection into a supra axillary plane [4].
A group of lateral axillary nodes may be concealed under the AA while crossing over the axillary vein. Missing these nodes during axillary node dissection imposes a risk for local recurrence in patients with breast cancer and melanoma. This also can lead to inaccurate staging, which in turn could negatively affect adjuvant and systemic therapy decisions for breast cancer [4].
The AA may be mistaken for the true lateral margin of the LD muscle. This may lead the surgeon to dissect in a plane above the axillary vein increasing the risk of injury to the axillary artery and brachial plexus [4]. During sentinel node biopsy, the AA can pose difficulty as it stretches in the hyper abducted position shifting the nodes higher [3].
In order to clearly identify, the anatomic landmarks the arch can be divided at the level of the axillary vein. Furthermore, some authors suggest division of the arch in all cases to prevent possible post-operative axillary vein compression and associated lymphoedema [3, 4]. In our case, there was no added morbidity related to dissection of nodes beneath and lateral to the arch.
The presence of the AA may precipitate lymphoedema in cases where LD myocutaneous flap is used for breast reconstruction. For this reason, the AA should be divided if there is a possibility of a LD flap being required in the future [3].
As in our case, most reported clinical cases describing the AA have been identified during axillary surgical procedures [2, 4]. The presence of AA has both clinical and surgical implications. It can cause confusion during routine axillary surgery for breast cancer, which can both affect procedure safety and misguide further treatment decisions [3, 4].
CONFLICT OF INTEREST STATEMENT
None declared.



