-
PDF
- Split View
-
Views
-
Cite
Cite
R. Kirby, N. Rajasagaram, M. Ghusn, Primary mesenteric gastrointestinal stromal tumour, Journal of Surgical Case Reports, Volume 2014, Issue 5, May 2014, rju050, https://doi.org/10.1093/jscr/rju050
Close - Share Icon Share
Abstract
Primary mesenteric gastrointestinal stromal tumours (GISTs) are rare tumours and can be included as a differential for an expanding intraabdominal mass. We present the case, in our institution, of a 72-year-old male who presented with non-specific symptoms and was diagnosed with a primary mesenteric GIST following resection. We report his follow-up and discuss the current theories as to the origins of these rare tumours and current treatment modalities.
INTRODUCTION
Gastrointestinal stromal tumours (GISTs) arise from mesenchymal stromal cells, interstitial cells of Cajal, and represent 5% of gastrointestinal tract malignancies. Cluster of differentiation molecules (CD117), a transmembrane receptor protein is essential to differentiate GIST from other mesenchymal masses [1]. Extragastrointestinal stromal tumours (EGISTs) are more common in people over 50 years old and originate in the omentum, peritoneum and mesentery [2].
CASE REPORT
We present a rare case of a 72-year-old male who presented to our institution with a 3-month history of increasing abdominal distension.
Our patient had no altered bowel habit, anorexia, nausea, vomiting or weight loss. He had no significant past medical or surgical history.
Workup included ultrasound-guided aspiration that yielded benign chronic inflammatory cells only. This was performed prior to our involvement in the patient's management and risk of seeding was a noted concern.
Computed topography (CT) of the chest, abdomen and pelvis reported a large mass predominantly cystic with more confluent solid-enhancing components and septation inferiorly and posteriorly measuring 21.7 cm (antero-posterior) × 16.3 cm (transverse) × 20.5 cm (infero-superior) in the upper abdomen. The mass appeared separate from the pancreas displacing small bowel loops with the lateral margin of the mass in contact and displacing the descending colon (Fig. 1).
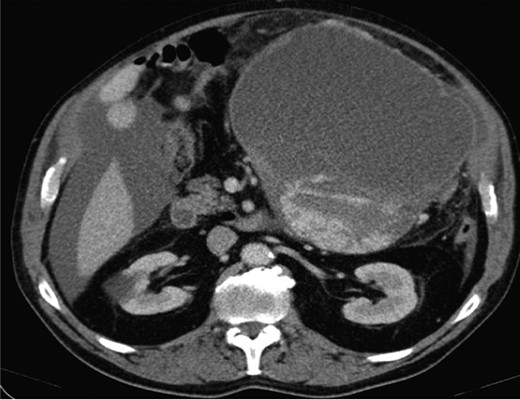
A laparoscopy identified a large cystic mass adjacent to the greater curvature of the stomach and a gastroscopy showed extrinsic compression alone. An endoscopic ultrasound reported a pseudocyst. The tumour markers were normal apart from a CA 125 219 (normal <35).
Intraoperatively, a large eight litre cystic mass was drained that was adherent to the greater curvature of the stomach (Figs 2 and 3).
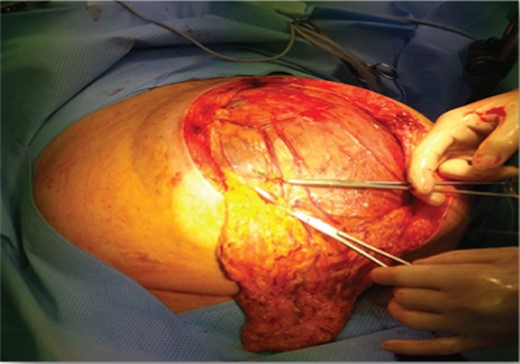
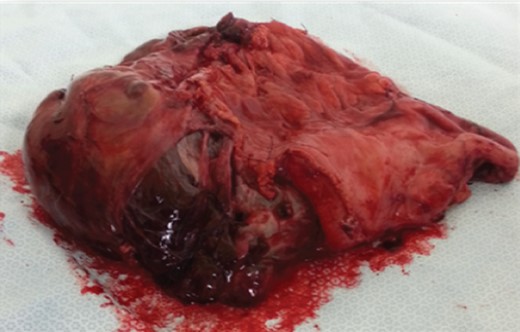
A distal gastrectomy with en bloc resection was performed. Histology reported a primary mesenteric GIST measuring ∼230 × 140 × 140 mm with complete local excision. The tumour showed patchy positivity for CD117 and diffuse extensive positivity for DOG-1. The mitotic rate was <5 per 50/hpf. The tumour cells were negative for smooth muscle markers, CD99, neural markers, PLAP, neuroendocrine markers, desmin and cytokeratins.
Following discharge and discussion at our multidisciplinary meeting imatinib mesylate was commenced. Follow-up was planned at 3 months with further CT scan of the abdomen and imatinib treatment for 9 months with further evaluation subsequently.
DISCUSSION
Primary mesenteric GISTs are EGISTs which are rare and currently there are two theories as to their origin.
Based on histology and immunophenotype EGISTs and GISTs are identical, hence EGISTs may represent GISTs that have separated from gastrointestinal tract or primary growths from the mesentery or omentum [3]. EGISTs have also been reported in the pleura, pancreas, abdominal wall and mesoileum.
More aggressive behaviour is based on mitotic rate >2/50 hpfs, Ki 67 >10%, cyst formation, necrosis and increased cellularity [4]. The National Institute of Health has developed a classification system for risk of malignant behaviour in GISTs, which ranged from very low risk to high risk and was based on tumour size and mitotic count [5] Currently, the mainstay of treatment of EGISTs is en bloc resection with imatinib [6–8].
A case reported combined neoadjuvant chemotherapy, surgery and adjuvant chemotherapy (epirubicin, cyclophosphamide and hydroxycamptothecin) that resulted in long-term survival [9] that may be useful in cases where there is resistance to imatinib. Following en bloc resection there is a 50% recurrence rate within 2 years [10] and as a result further research into these rare tumours is required.
CONFLICT OF INTEREST
We declare no conflict of interest. The corresponding author is not a recipient of a scholarship. The patient has granted permission for the images to be utilized. These images have not been submitted or published elsewhere. The paper has not been presented to a society or meeting. The co-author has contributed and is in agreement with its content.



