-
PDF
- Split View
-
Views
-
Cite
Cite
Jonathan Cook, Tanner Spees, Phillip Telefus, Jeffrey M. Ranaudo, Stephen Carryl, Philip Xiao, Pancreatic sarcoidosis discovered during Whipple procedure, Journal of Surgical Case Reports, Volume 2013, Issue 4, April 2013, rjt016, https://doi.org/10.1093/jscr/rjt016
Close - Share Icon Share
Abstract
Pancreatic sarcoidosis is a rare variant of systemic sarcoidosis, with cases described in literature as recently as January 2010. We present here a case of pancreatic involvement with non-caseating granulomas discovered on laparotomy in a patient with a preoperative diagnosis of pancreatic carcinoma. Computer tomography scan without contrast revealed a well-marginated smooth-shaped tumor in the head of the pancreas morphologically consistent with malignancy. During Whipple procedure, the mass was found to be a large lymph node that contained numerous non-caseating granulomas. Radiologically and clinically, non-caseating granulomas of the pancreas are often misdiagnosed as malignant tumor. Special attention given to this differential diagnosis by surgeons, pathologists and clinicians can avoid misdiagnosis and unnecessary treatment.
INTRODUCTION
Sarcoidosis is a multisystem disease of unknown etiology that can affect any organ. It is characterized by nodular noncaseating granulomatous lesions involving the lungs, skin, eyes, salivary glands and internal organs. It commonly affects adults from 20 to 40 years old, but can occur at any age. Most studies demonstrate a slightly higher incidence in women [1]. The incidence ranges from 11 per 100 000 in the Caucasian US population to 36 per 100 000 African Americans [1, 2]. The definitive etiology of sarcoidosis remains unknown. There is increasing evidence for multiple causative agents; however, association with a single agent or genetic locus remains elusive. The current model involves exposure to an antigen followed by an inordinate cell-mediated immune response [1, 3, 4]. Patients with sarcoidosis appear to have enhanced antigen presentation by HLA class II-bearing macrophages, inducing a T-helper cytokine response that leads to granuloma formation. The variety of sarcoidosis phenotypes, each associated with a different HLA class II molecule, supports the hypothesis that multiple inciting antigens processed by different T-cell clones can act as etiologic agents [4].
CASE REPORT
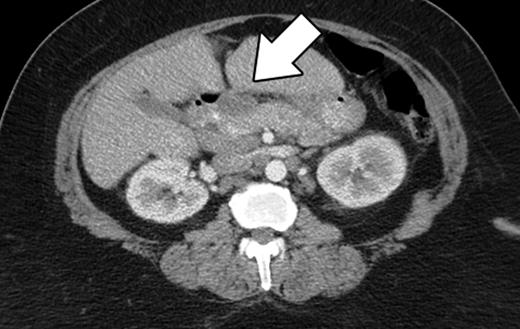
The patient is an asymptomatic 60-year-old African American female, who was referred to our institution for an elevated CA19-9. Her past medical history included diabetes mellitus, asthma, hypertension, hypercholesterolemia, obstructive sleep apnea and osteoarthritis. She denied abdominal pain, decreased appetite, nausea, vomiting, diarrhea, pruritus, jaundice, acholic stool or dark urine. She had no fevers, night sweats or other constitutional symptoms. She denied alcohol or tobacco use. Her family history was significant for colon and breast carcinoma. Her vital signs were within normal limits. She had no lymphadenopathy on examination of the head and neck. Abdominal exam revealed an obese, soft, non-tender, non-distended abdomen with no hepatosplenomegaly. The cardiovascular, neurologic and pulmonary exams were unremarkable. Computer tomography (CT) scan of her abdomen revealed a smooth shaped lesion involving the pancreatic head, measuring 3.6 cm at its greatest diameter (Fig. 1). Mesenteric lymphadenopathy was also noted, with the largest node measuring 2.3 cm in diameter. The liver, spleen, gallbladder and cystic duct were normal. These CT findings were consistent with pancreatic neoplasm and prompted surgical referral. After consultation, the patient agreed to a Whipple procedure. An endoscopic ultrasound (EUS) or fine needle aspiration biopsy was not preformed preoperatively.
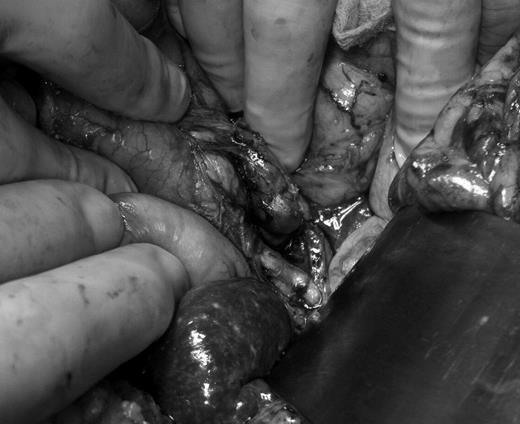
An upper midline laparotomy for a classic Whipple procedure was performed. After mobilization of the transverse colon, the pancreas and duodenum were visualized. On the mesentery near the pancreas there were several subcentimeter nodes which were excised and sent for frozen section. On the posterior surface of the pancreatic head, a large peripancreatic lymph node was identified and excised (Fig. 2). Intraoperative frozen section showed non-caseating granuloma, at which time the decision was made to abort the procedure. Postoperatively the patient had a smooth recovery. Drains were removed on post-op day 3 with minimal output. She spent an additional 2 days on the ward, after which she was discharged.
On gross pathological examination the specimen contained one peripancreatic lymph node, measuring 2.5 × 2.5 × 2 cm. Microscopic examination revealed normal lymph nodal effacement by granulomas composed of epithelioid cells with scattered Langhans giant cells and lymphocytes. Ziehl–Neelsen stain for acid fast bacilli (AFB) was negative. Grocott's methenamine silver stain for fungus was negative.
DISCUSSION
The presentation of sarcoidosis varies greatly, involving single organs or exhibiting widespread disease. In more than 90% of patients, sarcoidosis manifests with pulmonary involvement, and in the USA, the majority of patients present with chronic respiratory complaints with or without constitutional symptoms [1, 3]. Gastrointestinal involvement is rare. Pancreatic sarcoidosis is quite rare and is frequently asymptomatic. In 2006, a literature review by Caceres et al. demonstrated 25 cases of surgically proven pancreatic sarcoidosis with 12 presenting as a pancreatic head mass [5]. An autopsy series in Japan over 28 years reported pancreatic involvement in only 2.1% of patients with sarcoidosis, with more than half of those being asymptomatic at the time of death [6]. Symptomatic disease is extremely rare and is usually due to pancreatic duct obstruction or diffuse parenchymal disease. When present, symptoms include abdominal pain, nausea, anorexia, weight loss and jaundice. Pancreatitis without an obstructive mass has been reported, and may reflect parenchymal involvement [7].
Benign pancreatic head masses are rarely identified preoperatively, with the most common lesion being chronic focal pancreatitis. Efforts to avoid abdominal surgery and its associated morbidity and mortality risks have improved with the implementation of newer studies such as EUS-guided fine-needle aspiration (FNA) cytology. Currently, there is no consensus on the best imaging modality for pancreatic evaluation. A prospective study of 156 patients in 2011 by Brimiene et al. concluded that ultrasonography is the superior initial diagnostic technique for differentiating chronic pancreatitis and adenocarcinoma [8]. Baron et al. demonstrated a negative predictive value of 88% using EUS-guided FNA in differentiating benign from malignant pancreatic masses; however, 6 of 10 patients with a benign FNA were later proved to have a malignant mass at surgery [9].
Even with a high clinical suspicion for benign pathology, ruling out malignancy as a cause for pancreatic head mass is challenging. A preoperative biopsy demonstrating noncaseating granulomas does not rule out malignancy, as granuloma formation can be seen in Hodgkin's and non-Hodgkin's lymphoma, epithelial and germ cell malignancies and pancreatic adenocarcinoma [10].
While the presence of benign pathology is encountered in nearly 10% of Whipple procedures for suspected pancreatic malignancy, surgical resection of pancreatic head masses remains the standard of care. We agree with previous reports suggesting maintaining an awareness of sarcoidosis as a potential nonmalignant cause of pancreatic masses in patients from high-risk demographics.
Radiologically and clinically, noncaseating granulomas of the pancreas are often mistaken as malignant tumor. Special attention given to this differential diagnosis by surgeons, pathologists and clinicians can avoid misdiagnosis and unnecessary treatment.



