-
PDF
- Split View
-
Views
-
Cite
Cite
J Kinyanjui, N Butler, AL Lambrianides, Large Intra-abdominal desmoid tumour: complete resection with preservation of function, Journal of Surgical Case Reports, Volume 2012, Issue 4, April 2012, Page 8, https://doi.org/10.1093/jscr/2012.4.8
Close - Share Icon Share
Abstract
Desmoid tumours are benign monoclonal myofibroblastic neoplasms arising from musculoaponeurotic stromal tissue. They infiltrate local tissue but have no known metastatic potential. The management of desmoid tumours is complicated by their unpredictable nature and rarity, which makes study into their behaviour, and therefore treatment, a challenge. We present a case of intra-abdominal desmoid tumour and discuss the recommended approach to management.
INTRODUCTION
Desmoid tumours are rare, locally aggressive tumours with no known potential for metastasis or differentiation, accounting for only 0.03% of all neoplasms (1,2).
There are three main types (3):
Spontaneous tumours – rare and occur mainly in the extremities and abdominal wall
Tumours associated with familial adenomatosis syndrome (Gardner's syndrome) – risk is 10-30 % in these patients, which is further increased in mutations between exon 1310 and 2011
Familial infiltrative fibromatosis (hereditary desmoid disease) – extremely rare and also associated with adenomatous polyposis coli gene mutations
We present a case of spontaneous intra-abdominal desmoid tumour in an otherwise well male with a focus on management approach.
CASE REPORT
A 46-year-old male presented with 10 day history of abdominal pain, nausea and vomiting. He denied change in bowel habit, anorexia or weight loss and had no significant past medical history. Clinical examination revealed a gentleman of lean body mass but with apparently good nutritional status. Vital signs were normal. A large, non-tender, firm smooth-surfaced abdominal mass was palpable in the lower midline.
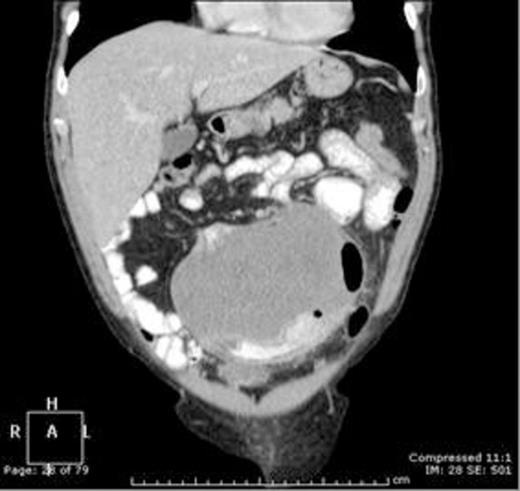
Coronal contrast enhanced CT scan of the abdomen and pelvis demonstrating an homogenous 14.6 x 11 x 14.3 cm intra-abdominal mass closely associated with the small bowel
Computed tomography (CT) (figure 1) showed a large well-defined tumour measuring 14.6 x 11 x 14.3cm arising from the mid-small bowel mesentery and closely associated with, or involving, the small bowel lumen. Abdominal ultrasonography confirmed the lesion to be solid and vascular. Subsequent magnetic resonance imaging (MRI) proved involvement of a jejunal segment and encasement of left-sided branches of the superior mesenteric artery. At laparotomy there was no evidence of synchronous disease. The tumour and associated jejunum with its vascular pedicle was resected in-toto (figures 2,3). Small bowel continuity was re-established with stapled side-to-side primary anastomosis. Post-operative course was uneventful.
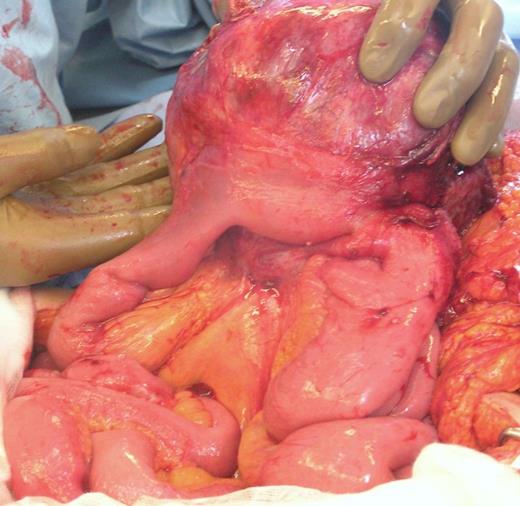
Intra-operative photograph showing tumour arising from small bowel mesentery with local involvement of jejunum
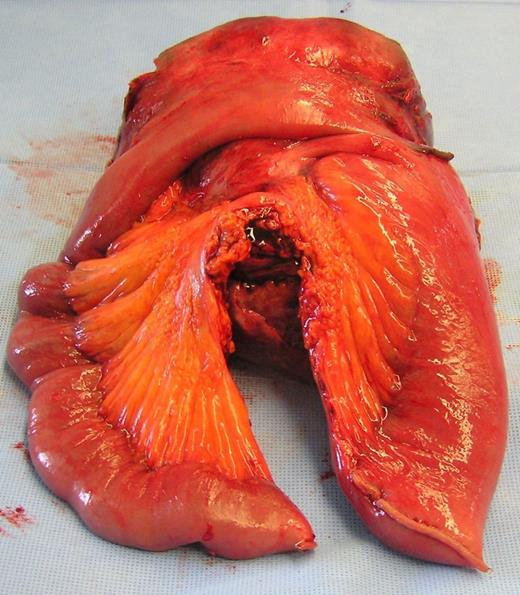
Post-operative photograph showing resected specimen with associated jejunum and its mesenteric pedicle in the foreground
On histological assessment the specimen showed bland spindled cells arranged in intersecting fascicles. Involvement included the small bowel mesentery and jejunal muscularis propria with focal extension into submucosa (figure 4). The microscopic margin of the tumour was ill defined but appeared clear of the resection limit. Immunohistochemistry showed strong nuclear expression for beta-catenin but was negative for C-KIT, CD34 and DOG1 consistent with a diagnosis of desmoid tumour (3,4,5).
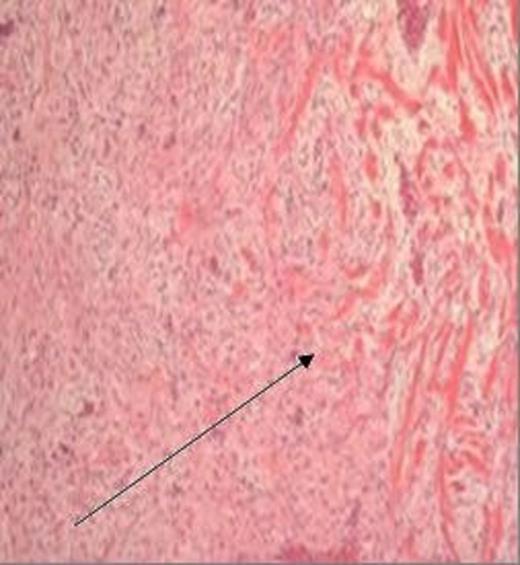
H&E stained section, magnification x 40, demonstrating a bland spindled cell lesion arranged in intersecting ill-defined fascicles, with some intervening collagen fibres and relatively limited infiltration at the lesion margin (arrow)
DISCUSSION
The rarity of intra-abdominal desmoid tumour precludes the use of randomised controlled trials to compare treatment options. Natural history of the disease is also variable: tumours may regress spontaneously, show long-term stability or behave aggressively. This further complicates the development of standardised treatment regimens and it remains unclear whether any intervention improves survival. Treatment must be tailored and a multidisciplinary approach is thus recommended. Current therapeutic modalities mirror those for most other neoplasms and include surgery, radiotherapy and systemic therapy.
In the case of isolated intra-abdominal desmoid tumour surgical resection provides the most successful primary treatment, however a number of patient and disease factors require consideration. The importance of excision margin is unclear because although positive margins after surgery reflect higher risk, recurrence rates even after complete resection are 16-39 percent (1,3). For isolated intra-abdominal disease the goal of surgery thus becomes complete resection but with maximal preservation of function to minimise morbidity (3). Surgery may also be indicated in the setting of multifocal disease where immediate threat to life or function exists. Attempted curative resection for intra-abdominal desmoid associated with Gardner’s syndrome is contraindicated because disease is always diffuse and poorly defined (6).
In cases where there is no immediate threat to life or vital organ function, or surgery is not technically feasible and/or contraindicated due to co-morbidity or multifocal disease, ionising radiation and systemic therapy should be considered. Such treatments may be employed in combination and can also provide adjunctive benefit before or after surgery.
Desmoid tumours are radiosensitive (1,7). Current guidelines from the national comprehensive cancer network suggest that post-operative radiotherapy be considered for large tumours and those with positive margins (3,7).
Systemic therapy should be considered for localised disease not otherwise amenable to potentially curative resection or radiotherapy, as neo- or adjuvant treatment for localised intra-abdominal tumours, and in the setting of multifocal disease. The goal of pharmacotherapy may be to induce remission, prevent complications, and/or reduce morbidity (8). The choice of agent depends on the urgency of the situation.
Cytotoxic agents generally provide the most rapid clinical response at the expense of increased systemic side-effects and are thus indicated where imminent (but not immediate) threat to life or function exists. Retrospective data from two separate studies regarding single versus combination therapy is contradictory (3,9). Different combinations of doxorubicin, ifosfamide and methotrexate have resulted in disease stabilisation rates 50-80% after six months (1).
Hormone modulators and non-steroidal anti-inflammatory drugs (NSAID’s) have lower adverse risk profiles and should be considered for all other presentations where surgery is precluded. Use of most non-cytotoxic drugs is based on case reports. Response rates of 50% have been reported, with regression rates of 10-15% and disease stabilisation or shrinkage in 25% (1,3). The selective oestrogen receptor modulator Tamoxifen is thought to act via ER-beta expressed on tumour cells, however the exact mechanism by which regression occurs remains unclear (6). The use of NSAIDs is based on a single case report where incidental total regression of tumour occurred in a patient taking indomethacin for radiation induced pericarditis (3). Success rates of between 37-57% have been reported with indomethacin and sulindac (3,8).
Other proven therapeutic targets include immunomodulation with interferon alpha, and disruption of cellular signal transduction with selective tyrosine kinase inhibitors. Interferon alpha provides an alternative to cytotoxic agents where tumour is refractory to NSAIDs and/or hormone therapy. The selective tyrosine kinase inhibitor Imatinib has been shown to achieve disease stabilisation rates at least comparable to established cytotoxic chemotherapeutic regimens (3).
Post-treatment surveillance for local recurrence involves clinical assessment and imaging of the tumour site every three to six months for the first two to three years, followed by annual follow-up thereafter (1,3). In the event of recurrence all treatment modalities should be reconsidered, preferably in a multi-disciplinary conference.
Our patient was discussed by such a multi-disciplinary team consisting of surgeons, medical and radiation oncologists, and a histopathologist. Surgery alone was recommended and the patient remains disease free six months following operative resection.



