-
PDF
- Split View
-
Views
-
Cite
Cite
NA Papakonstantinou, G Hardavella, G Papavasileiou, N Anastasiou, Medical thoracoscopy for the treatment of complicated hepatic hydrothorax, Journal of Surgical Case Reports, Volume 2012, Issue 3, March 2012, Page 2, https://doi.org/10.1093/jscr/2012.3.2
Close - Share Icon Share
Abstract
Hepatic hydrothorax is a complication of liver cirrhosis. Several invasive therapeutic approaches have been performed such as thoracentesis, video-assisted thoracic surgery and repair of the diaphragmatic defects with debatable results. Medical thoracoscopy is a minimally invasive and effective technique with challenging applications that has not been thoroughly used in the treatment of hepatic hydrothorax. We hereby present the case of a hepatic hydrothorax complicated by thoracic empyema in a 38-year-old patient with alcoholic cirrhosis that was treated with medical thoracoscopy and talc pleurodesis.
INTRODUCTION
Hepatic hydrothorax (HH) is a complication of liver cirrhosis in patients with ascites. It is defined as a significant pleural effusion (>500ml) in cirrhotic patients without primary cardiac or pulmonary disease (1). It occurs in approximately 4-6% of cirrhotic patients (1). There are no guidelines on therapy of HH based on good evidence, thus the optimal management remains unclear (2). Available options include sodium restriction and diuretics, thoracentesis, video-assisted thoracic surgery (VATS) with talc pleurodesis, repair of the diaphragmatic defects, transjugular intrahepatic portosystemic shunts, pleurovenous shunting and liver transplantation (3–5). We hereby present the case of a 38-year-old patient with alcoholic cirrhosis who was diagnosed with HH complicated by thoracic empyema. The patient was treated with medical thoracoscopy (MT) and talc pleurodesis. Informed consent was obtained.
CASE REPORT
A 38-year-old man with a background history of alcoholic cirrhosis was referred to our department with HH one year ago. Two weeks before that, the patient was admitted in a secondary health care setting due to sudden dyspnea and ascites. The chest X-Ray (CXR) showed massive pleural effusion in the left hemithorax and a chest tube was erroneously inserted and drained transudative fluid (˜3l/day) (Figure 1a). The patient was diagnosed with HH. On day 10, the patient presented with fever; he was treated with antibiotics and was referred to our department for further management of his HH.
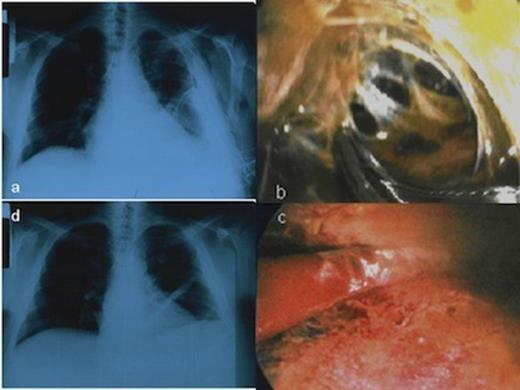
CXR showing the HH and the chest tube in the left hemithorax before the MT, b. Multiloculated thoracic empyema as a complication of HH, c. Disruption of fibrinous adhesions and debridement of parietal and visceral pleura, d. Chest X-ray following MT showed no recurrence of the hydrothorax
The patient was classified as Child-Pugh C category. Heart ultrasound was normal. We decided to perform talc pleurodesis by MT with local anesthesia in order to avoid potential hepatotoxic effects caused by drugs used in general anesthesia. Local anesthesia was performed with lidocaine and ropivacaine hydrochloride while midazolam, fentanyl citrate and propofol drips were used for sedation. The chest tube was removed and the thoracoscope entered the pleural cavity through a port. One additional incision (~1 cm) was made for instruments’ insertion. Surprisingly, inspection of the pleural cavity revealed multiloculated thoracic empyema. Fibrinous adhesions were disrupted and parietal and visceral pleura were debrided in order to unify the pleural space. The complicated pleural effusion was evacuated (Figure 1b, c). A segment from parietal pleura was sent for culture and pleural fluid was also sent for culture and biochemical analysis. Finally, talc pleurodesis was performed, so the lung parenchyma re-expanded with obliteration of the pleural cavity. Postoperatively, the patient had leukocytosis and fever. Pleural fluid was a transudate infected by Staphylococcus aureus sensitive to azithromycin which was administered to the patient. CXR following MT showed no recurrence of the hydrothorax (Figure 1d). Figure 2 shows the gradual improvement of patient's serum test levels during his hospital stay. The patient was discharged on the 8th postoperative day in good condition and did not have any recurrence in a follow-up of 4 months after the procedure.
DISCUSSION
HH is usually a right-sided transudative pleural effusion although in 12.5% of cases it can be left-sided, like in our case (1). Despite the existing variety of literature regarding the treatment of symptomatic HH, optimal therapy still remains vague.
In our case, a chest tube was initially inserted to the patient. Chest tube insertion can be very dangerous in patients with HH. De Campos et al (4) reported high morbidity and mortality due to associated fluid and protein losses. Complications (increased bleeding due to coagulopathy, infection secondary to poor wound healing, renal failure, and electrolyte disturbances due to fluid losses etc) are more frequent in patients with cirrhosis having a chest tube. Consequently, this procedure should be avoided whenever possible (5).
To the best of our knowledge, this is the first reported case of a complicated HH treated with talc pleurodesis by MT. MT proved to be equally efficient to VATS and less invasive.
VATS, the least minimally invasive procedure performed so far for the treatment of HH, allows detection and closure of diaphragmatic defects simultaneously with talc pleurodesis. It has a mortality rate of 3% and has been so far an excellent diagnostic and therapeutic tool for HH (6). Reported rates of effectiveness of pleurodesis with talc poudrage range from 43.7% to 80% (2,4,7). If VATS is repeated, these rates range from 73% to 80% (6). Even higher rates were presented by Lin et al (6). They reported complete response in 14/24 patients that underwent pleurodesis via thoracoscope and partial response in 8/24 patients. During the follow-up period (6 months to 3 years), no procedure-related mortality or morbidity was reported while only 1 patient recurred in 18 months (7). Similar results were reported by Aelony et al (8) who evacuated pleural effusions in 34/39 patients (87%) without procedure-related mortality or morbidity.
VATS requires general anaesthesia with selective endobronchial intubation and at least three points of entry. General anesthesia would be potentially hepatotoxic for our cirrhotic patient while the minimum of three entry points would be dangerous due to coagulation disorders. We performed MT with local anaesthesia, conscious sedation and only two points of entry that was less invasive and offered numerous profits: (a) avoidance of endotracheal intubation and general anaesthesia in a cirrhotic patient with coagulation disorders (b) section of adhesions in the empyema (c) drainage of complicated HH (d) evaluation of the lung re-expansion capacity (e) better immediate and late postoperative patient condition, (f) less deterioration of lungs function, (g) tissue collection for histologic and microbiologic analysis, (h) reduction in postoperative hospital stay and no need for intensive care unit.
Moreover, talc poudrage insufflation by MT has optimal results in pleurodesis (8) and less side effects than tetracycline/doxycycline/povoiodine (9). Our procedure was efficient due to complete patient response and non recurrency at follow up.
MT is a safe, efficient and minimally invasive technique. When performed by trained physicians and contraindications are well considered, it has slight complications, minor mortality and 0.6% morbidity (10). It constitutes a novel, less invasive, effective and safe procedure revealing exciting possibilities in everyday clinical practice.



