-
PDF
- Split View
-
Views
-
Cite
Cite
A Ali, A Krishnan, S Rehman, VSR Rao, H J Pearson, Giant colonic mucocele following palliative surgery for metastatic adenocarcinoma, Journal of Surgical Case Reports, Volume 2011, Issue 3, March 2011, Pages 1–4, https://doi.org/10.1093/jscr/2011.3.9
Close - Share Icon Share
Abstract
We report an unusual case of a giant colonic mucocele following ileo-sigmoid bypass surgery in a patient with advanced adenocarcinoma of the splenic flexure. The formation of a giant colonic mucocele resulted from distal splenic flexure obstruction due to tumour relapse and proximal caecal obstruction due to peritoneal disease with subsequent accumulation of mucus in the closed loop.
CASE REPORT
A 73 year old female patient presented in May 2005 with lower abdominal pain, abdominal bloating and increased frequency of bowel habits. Subsequent colonoscopy detected a neoplastic lesion at the splenic flexure confirmed at biopsy to be adenocarcinoma. CT scan showed no evidence of metastatic disease and primary tumour was deemed resectable. However, at laparotomy, the primary arising from the splenic flexure was found to be advanced with diffuse intra abdominal metastases. Hence a palliative bypass was fashioned in the form of an ileo sigmoid anastomosis. Peritoneal biopsies confirmed invasive adenocarcinoma. Post-operatively, the patient received palliative chemotherapy with good response indicated by tumour shrinkage in serial CT scans and falling carcinoembryonic antigen (CEA).
Subsequently, in March 2007, the patient developed weight loss and abdominal pain. CT revealed liver metastasis and local relapse at the splenic flexure. She was commenced on second line chemotherapy. She later presented to the emergency department in August 2007 with abdominal pain and marked abdominal distension. Physical examination revealed abdominal distension with no features of peritonism or intestinal obstruction. Abdominal X-Ray revealed distended bowel loops (Figure 1). She was initially managed conservatively. Due to lack of clinical improvement, it was decided to proceed to laparotomy for large bowel obstruction.
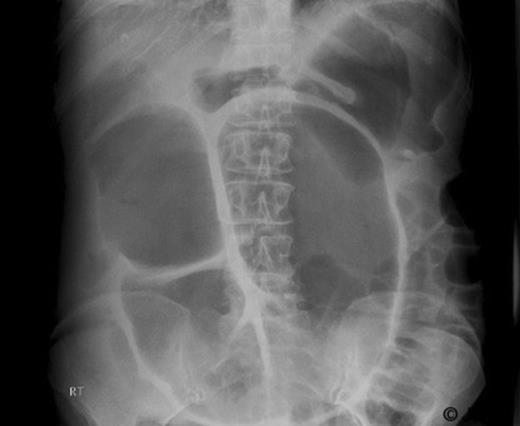
At laparotomy, the ascending and transverse colon was dilated to 12 cm with features of a giant mucocele which yielded 3.5 litres of clear mucus. This was found to be due to closed loop obstruction between the terminal ileum adherent to the pelvis as a result of peritoneal disease and local relapse at the splenic flexure resulting in accumulation of mucus and formation of a mucocele. A loop ileostomy was fashioned and the transverse colon was drained with a 24 French 2 way catheter brought out at the top end of the wound. Post operatively, about 1600 ml of mucus was drained from the catheter in the initial few days and this subsequently reduced to 125 ml daily. The catheter was subsequently removed after 6 weeks.
DISCUSSION
Appendiceal mucocele is a well recognized entity and occurs due to appendiceal obstruction followed by luminal accumulation of mucus and dilatation of the obstructed appendix (1). It may be associated with concomitant colon cancer and hence thorough investigation of the colorectal tract is recommended after diagnosis of an appendiceal mucocele (2). Rarely an appendiceal mucocele can become very large and can present as an abdominal mass (3,4).
Similarly, colonic mucoceles can very rarely occur due to a closed loop obstruction. It has been reported as an unusual complication after diversion transverse loop colostomy in a patient with long-standing ulcerative colitis resulting from distal stomal and rectal stenosis and accumulation of mucus in the closed loop over many years 5). Mucocele following sequestration of a segment of bowel wall at the site of colo-colic anastomosis after resection of sigmoid carcinoma has been reported. In this case, the patient had rising carcino-embryonic antigen levels (CEA) with increased uptake on positron emission tomography (PET) scan at the site of original anastomosis raising the suspicion of a recurrence. Following excision of the mucocele, CEA levels dropped to normal (6). Perforation of a colonic mucocele has been reported 22 years after formation of ileostomy in a patient with Crohn’s disease. This occurred due to stenosis of the distal colonic segment and subsequent development of a mucocele (7).
In this case, a closed loop of the right colon and transverse colon was formed by distal obstruction at the splenic flexure by tumour relapse and proximal obstruction at the ileo-caecal valve by peritoneal metastasis. The continued secretion and accumulation of mucus led to distension of the closed colonic loop. A similar presentation has been reported in a patient with carcinomatosis peritonei from recurrent squamous cell carcinoma of the cervix, eight months after palliative bypass of obstructed ascending colon (8).
Colonic mucoceles mostly present with abdominal distension which usually evolve slowly and insidiously over a period of 2-6 years (5). It may cause obstructive symptoms if it is large enough to compress adjacent bowel segments. In patients with loop colostomies or mucous fistulas, the cessation of mucous drainage from either the rectum or the mucous fistula should alert the surgeon to the possibility of a closed loop obstruction (9). Imaging modalities such as ultrasound and CT can be used to aid diagnosis and this requires a high index of suspicion.
In a majority of cases, the diagnosis is confirmed at exploratory laparotomy. In this case, a CT scan done five months before the acute presentation revealed colonic dilatation and local relapse at the splenic flexure along with liver metastasis and peritoneal disease (Figure 2). A diagnosis of colonic mucocele was not made and the air fluid level in the closed loop was thought to be due to competent ileocaecal valve. On hindsight, the absence of intestinal obstruction in spite of impressive colonic dilatation in the CT scan should have raised the suspicion of an evolving colonic mucocele. This reinforces the need for a high index of suspicion so that the diagnosis can be made pre-operatively and the patient managed conservatively.
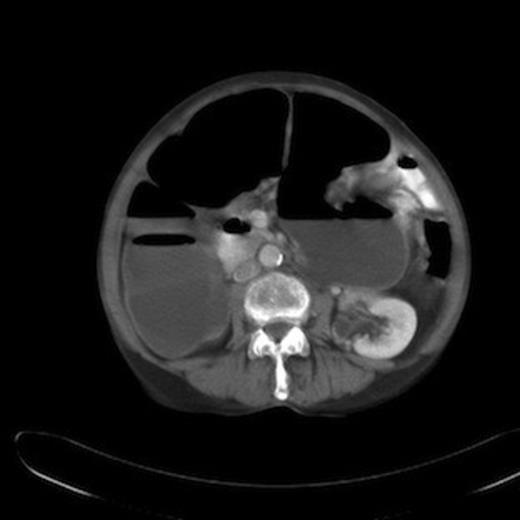
CT abdomen showing dilatation of ascending and transverse colon upto splenic flexure with air fluid levels.
In a case where diagnosis has been confirmed on imaging, treatment can be undertaken by CT guided drainage (8). Other treatment options include excision of the mucocele and this should usually be undertaken in low-risk surgical patients who have a good prognosis (5). In cases where there is a high chance of mucocele recurrence, a permanent catheter or a sump drainage tube can be placed and the patient can be taught self irrigation (5).
CONCLUSION
Colonic mucocele is an extremely rare entity and requires a high index of suspicion for diagnosis. The treatment depends on the underlying disease process and the overall prognosis of the patient. We believe this to be the first reported case in literature of a colonic mucocele following palliative bypass surgery for advanced colonic cancer.



