-
PDF
- Split View
-
Views
-
Cite
Cite
Anya Adair, Anca Oniscu, Stephen J Wigmore, Post-surgical Pancreatitis Masquerading as Recurrent Neuroendocrine Cancer, Journal of Surgical Case Reports, Volume 2010, Issue 4, June 2010, Page 1, https://doi.org/10.1093/jscr/2010.4.1
Close - Share Icon Share
Abstract
Neuroendocrine tumours of the pancreas can have a spectrum of behaviour from relatively benign to aggressive. Resection can result in cure although metastatic disease is described. We present an unusual case of an apparent local recurrence of previously resected neuroendocrine tumour in a young man who had undergone distal pancreatectomy. Pathological analysis demonstrated focal post-surgical pancreatitis with radiological appearances bearing striking similarity to the original primary tumour.
INTRODUCTION
Neuroendocrine tumours of the pancreas have previously presented in association with both acute and chronic pancreatitis (1,2,3). By contrast pancreatitis presenting as a malignant neuroendocrine tumour has never been reported.
We report a case of post-surgical pancreatitis presenting clinically as recurrent neuroendocrine tumour of the pancreas.
CASE REPORT
A 42 year old man presented with a history of flushing and sweats and was found on investigation to have a lesion associated with the body of his pancreas. His gut hormone profile was normal and urinary 5-hydroxy indole acetic acid levels were similarly within the normal range. He underwent biopsy of the lesion via endoscopic ultrasound which confirmed the presence of a neuroendocrine tumour of carcinoid subtype. He underwent a spleen preserving distal pancreatectomy for a carcinoid tumour situated in the midbody of the pancreas (fig1).
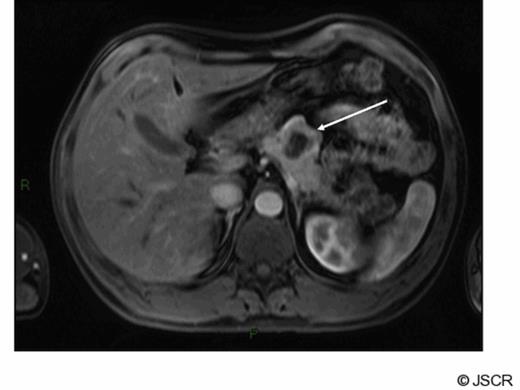
Arterial phase gadolinium enhanced MRI demonstrating primary tumour (arrow)
At operation this was a well defined lesion with no evidence of intra-abdominal metastasis. There was no evidence of pancreatitis and the remaining gland appeared normal. Pathology confirmed a single well demarcated mass that histologically was a well-differentiated endocrine tumour. No lymphovascular space invasion was noted and complete excision was achieved with good margins. The tumour measured 39mm, the Ki-67 proliferation index was less than 2% and together with a mitotic count of less than 1 in 10 high powered fields confirmed this was, according to the WHO classification, a well-differentiated (grade 1) endocrine tumour of uncertain behaviour. No cytological atypia or necrosis was identified. The patient made an uneventful post operative recovery however at follow up 6 months later he complained of recurrent sweats and hot flushes. CT scan showed apparent evidence of recurrence of tumour at the site of the previous resection margin in a similar location to the original primary tumour with additional abnormal soft tissue adjacent to the greater curvature of the stomach consistent with local or nodal recurrence. No liver or lung metastases were identified. These findings were confirmed on MRI scan (fig 2).
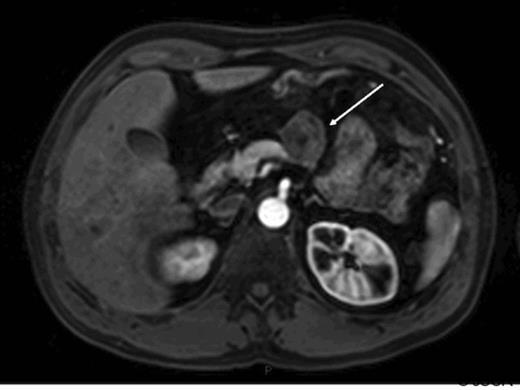
Arterial phase gadolinium enhanced MRI demonstrating lesion reported as being consistent with recurrent neuroendocrine cancer (arrow).
Laparotomy was performed which showed what appeared to be recurrent tumour at the anterior aspect of the pancreas infiltrating through the posterior wall of the stomach over a significant surface area with additional tumour deposits in the transverse mesocolon. A subtotal gastrectomy with Roux-en-Y reconstruction, transverse colectomy, resection of body of pancreas and splenectomy were carried out. The patient made a good recovery. Subsequent pathology showed similar macroscopic and microscopic features for all the lesions described. These were necrotic pseudocystic structures encased within inflamed fibrous tissue. There was surrounding fat necrosis, giant cell reaction and haemosiderin-laden macrophages were prominent. The inflammation expanded to involve the wall of stomach explaining the intraoperative appearance. These findings were consistent with subclinical post-surgical pancreatitis. No histological evidence of a residual endocrine neoplasm or other malignancy was identified.
DISCUSSION
Malignant non functioning neuroendocrine tumours of the pancreas are uncommon. They often present late with weight loss and abdominal pain being their most common clinical manifestations (4,5). Their prognosis is considerably better than for adenocarcinoma of the pancreas. Resection is the treatment of choice. A negative resection margins is a favourable prognostic indicator (5,6,7) however, recurrence following curative resection has been reported to be as high as 20% (8). Other factors indicating a favourable outcome include small tumour size, absence of lymph node invasion and distant metastases.
Various pancreatic masses have in the past been reported as mimicking pancreatic cancer with radiological studies not always successful in differentiating the aetiology of a pancreatic mass (9). In addition both chronic pancreatitis and peripancreatic fat necrosis following acute pancreatitis have been misdiagnosed as pancreatic malignancies both on clinical and radiological findings with the correct diagnosis only confirmed on surgical pathology or percutaneous biopsy with prolonged follow up showing no evidence of malignancy (10,11,12). Reports have suggested that 18F-fluordeoxyglucose scanning is a highly sensitive and specific method to differentiate between benign and malignant masses including neuroendocrine tumours (13). It would also have been possible to use either radionucleotide Octreotide or Meta-iodobenzylguanidine (MIBG) scanning to try and improve the accuracy of diagnosis of neuroendocrine carcinoma. These receptor ligand assays can be very useful in a diagnostic setting but are not always positive in patients with neuroendocrine cancer.
This patient's initial presenting symptoms were flushing and night sweats despite never having any liver metastases. In addition gut hormone and 5HIAA levels were normal. It was recurrence of these symptoms post operatively which prompted further investigations.
It was unusual that this patient's neuroendocrine tumour appeared to have recurred so aggressively and so promptly following resection. This is particularly so since the primary tumour had been a circumscribed well-differentiated endocrine neoplasm excised with a clearance of 7.5mm. However, the CT and MRI results were so compelling for tumour recurrence that tumour recurrence could not be excluded and certainly at operation the tissue characteristics were classical of tumour deposits.
Prior to his initial surgery he had never had elevated 5HIAA urinary levels and therefore this was not a useful marker to assess possible recurrence.
Laparoscopy would have been of no benefit in characterising the lesions and with significant necrotic tissue and a severe inflammatory reaction it would have been difficult to differentiate on frozen section.
With such convincing findings both on imaging and at surgery the diagnosis could only be made with any reassurance following resection.



