-
PDF
- Split View
-
Views
-
Cite
Cite
Chia-Hung Chien, Yi-Fan Chiang-Hsieh, Yi-An Chen, Chi-Nga Chow, Nai-Yun Wu, Ping-Fu Hou, Wen-Chi Chang, AtmiRNET: a web-based resource for reconstructing regulatory networks of Arabidopsis microRNAs , Database, Volume 2015, 2015, bav042, https://doi.org/10.1093/database/bav042
Close - Share Icon Share
Abstract
Compared with animal microRNAs (miRNAs), our limited knowledge of how miRNAs involve in significant biological processes in plants is still unclear. AtmiRNET is a novel resource geared toward plant scientists for reconstructing regulatory networks of Arabidopsis miRNAs. By means of highlighted miRNA studies in target recognition, functional enrichment of target genes, promoter identification and detection of cis- and trans- elements, AtmiRNET allows users to explore mechanisms of transcriptional regulation and miRNA functions in Arabidopsis thaliana , which are rarely investigated so far. High-throughput next-generation sequencing datasets from transcriptional start sites (TSSs)-relevant experiments as well as five core promoter elements were collected to establish the support vector machine-based prediction model for Arabidopsis miRNA TSSs. Then, high-confidence transcription factors participate in transcriptional regulation of Arabidopsis miRNAs are provided based on statistical approach. Furthermore, both experimentally verified and putative miRNA-target interactions, whose validity was supported by the correlations between the expression levels of miRNAs and their targets, are elucidated for functional enrichment analysis. The inferred regulatory networks give users an intuitive insight into the pivotal roles of Arabidopsis miRNAs through the crosstalk between miRNA transcriptional regulation (upstream) and miRNA-mediate (downstream) gene circuits. The valuable information that is visually oriented in AtmiRNET recruits the scant understanding of plant miRNAs and will be useful (e.g. ABA-miR167c-auxin signaling pathway) for further research.
Database URL:http://AtmiRNET.itps.ncku.edu.tw/
Introduction
MicroRNAs (miRNAs) are a class of small (21–24 nucleotides) post-transcriptional gene silencers extensively found in plants and animals. Over the past decade, trends of miRNA research have been highlighted in following disciplines after the discovery of novel miRNAs. Target recognition, either computational prediction or experimental confirmation, is a dominant strategy to explore mRNAs that are hybridized by a specific miRNA. The biological protagonists of miRNA targets facilitate the inference of miRNA functions, especially for those with plenty of target genes or miRNA targets acting as hubs of genetic regulatory circuits enriched in cellular and developmental processes, biochemical secretion and pathogenesis ( 1 , 2 ). Additionally, studies on transcriptional regulation of miRNA reinforce the understanding of fundamental mechanism that contributes miRNA expression ( 3 ). Not only the characteristics of miRNA genes (e.g. gene length, secondary structure and promoters) but also what transcription factors (TFs) participate in miRNA regulation under specific conditions or environments were promptly unveiled. Among them, accurately identifying miRNA promoters is an essential prerequisite for large-scale investigation of TF-miRNA regulatory relations.
Owing to the importance of up-to-date miRNA studies mentioned above for understanding regulatory mechanisms and functions of miRNAs comprehensively, this work aims to integrate these research fields and reconstruct regulatory networks of Arabidopsis miRNAs ( Figure 1 ). Compared with animals, plant miRNAs are rarely investigated both in functional annotation and transcriptional regulation. Arabidopsis thaliana was chosen in this study due to its complete genome reference and abundant data sources. To elucidate miRNA-target interactions (MTIs) in Arabidopsis , we collected experimentally verified targets as well as their indirect targets, which were unprecedentedly considered. The putative targets were also provided, combining with confirmed MTIs, to perform enrichment analysis of gene ontology (GO) terms. On the other hand, the reported support vector machine (SVM)-based prediction model successfully identified miRNA transcriptional start sites (TSSs) in human ( 4 ). However, it may not be applicable for plants. To address the incompetence, we created a TSS prediction model specific to plants for identifying Arabidopsis miRNA TSSs. Based on the high-confidence TSSs, promoters of Arabidopsis miRNA were acquired to discover cis- and trans- elements using the improved concept we published previously ( 5 ). Finally, a web-based resource, AtmiRNET, was established to visualize inferred regulatory networks of Arabidopsis miRNAs through the integration of informative relations.
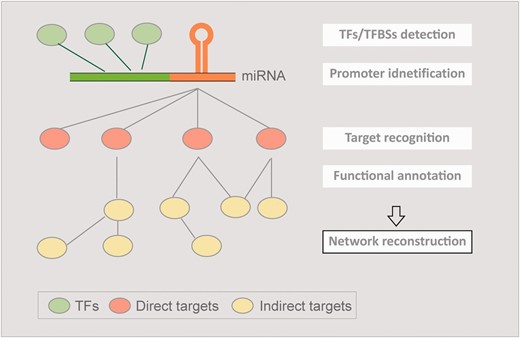
The concept of AtmiRNET. AtmiRNET summarizes the means of up-to-date miRNA research focusing on target recognition, functional enrichment of targets, promoter identification, detection of cis- and trans- elements to infer regulatory networks of Arabidopsis miRNAs, which are effective to augment the understanding of miRNA functions and transcriptional regulation.
Materials and Methods
Establishing SVM-based model to identify Arabidopsis miRNA promoters
The chromosomal coordinates and annotations of 290 Arabidopsis pre-miRNAs were obtained from miRBase release 18 ( 6 ). The 10 kb upstream of the pre-miRNAs were retrieved for analysing putative TSSs. Moreover, experiment verified TSSs were collected from RIKEN database, which contains 12 041 unique TSSs of Arabidopsis protein-coding genes ( 7 ). Only genes with unique TSS were considered. Due to the lack of TSS-relevant high-throughput sequencing data on chloroplast and mitochondrion, genes locate on the chloroplast and mitochondrial DNA were excluded.
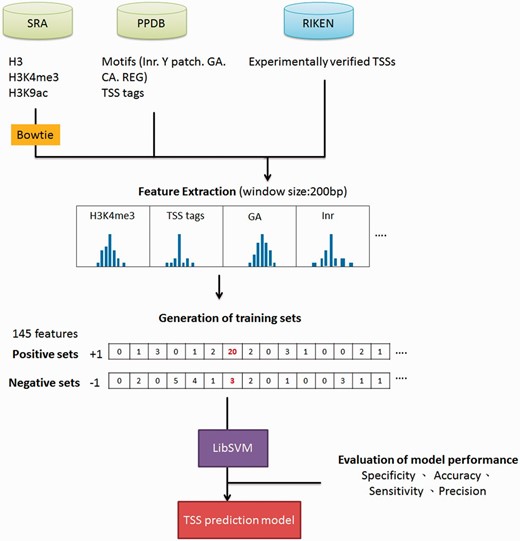
Datasets used for training promoter prediction model are summarized in Supplementary Table S2 . ChIP-seq data for H3K4me3, H3K9ac and nucleosome H3 were accessed via SRA accession number SRR058388, SRR058387 and SRR052645, respectively. Additionally, TSS tags derived from sequencing of full-length cDNAs were downloaded from PlantPromDB (PPDB) ( 8 ). Bowtie was used for mapping raw reads of high-throughput sequencing datasets to Arabidopsis genome ( 9 ). Only unique alignments were retained for further analysis. Information of core promoter motifs were obtained from PPDB as well, including pyrimidine patch (Y Patch), initiator (Inr), CA and GA elements, and regulatory element groups (REGs) ( 8 , 10 ). For the supporting evidence of TSSs, Arabidopsis expressed sequence tags and conservations among seven plants using phastCons method were downloaded from TAIR ( 11 ) and http://gregorylab.bio.upenn.edu/arabidopsisStructure/conservation , separately. Finally, the SVM-based model used to identify Arabidopsis miRNA TSSs was created by following the methodology we published previously ( 4 ). Not only the performance (sensitivity, specificity, accuracy and precision) of prediction model was evaluated by 5-fold cross-validation (CV), 63 experimentally verified miRNA TSSs from the previous study were also used for independent test ( 12 ).
Detection of cis - and trans -regulatory elements for Arabidopsis miRNA genes
The coTFBS represents the common TFBS motifs coincide in promoters of a miRNA and its coexpressed annotated genes. In this work, we combined the coexpression analysis with hypergeometric P -values to obtain statistical significance of over-represented TFBSs in Arabidopsis miRNA promoters.
First, the expression profiles of Arabidopsis miRNAs and annotated genes were retrieved from mirEX and AtGenExpress representatively ( 13 , 14 ). Since mirEX only exhibits the expression profiles of 190 miRNA precursors under 11 development stages in Arabidopsis , miRNAs with no expression information were discarded. For Arabidopsis annotated genes, developmental series of GSE5629, GSE5630, GSE5631, GSE5632, GSE5633 and GSE5634 containing 22 746 genes (probe sets) were accessed from Gene Expression Omnibus ( 15 , 16 ). After the normalization process between these expression datasets, Pearson’s correlation coefficient (PCC) and Spearman’s rank correlation coefficient were applied to estimate which annotated genes are co-expressed with specific miRNAs.
Then, 1000-bp long promoters (FASTA format) of 281 Arabidopsis miRNA precursors were acquired according to either the experimentally verified TSSs or putative ones identified by SVM prediction model in AtmiRNET. For detecting all possible TFBSs harbored in promoter regions of miRNAs and their coexpressed annotated genes, Match program ( 17 ) was executed for searching sequence motif of TFBSs using the matrix information from TRANSFAC Spring Release 2014.1 and position weight matrices (PWMs) from the recent literature ( 18 ).
Recognition of Arabidopsis miRNA targets and their function annotation
Putative and experimentally verified Arabidopsis MTIs are derived from psRNATarget and miRTarBase representatively ( 19 , 20 ). The correlations between the expression levels of miRNAs and their targets are also calculated by PCC. Moreover, AtmiRNET provides the information of indirect targets, a group of genes that are indirectly mediated by a specific miRNA, that is, the downstream gene circuits of miRNA direct targets. To distinguish full downstream genes of miRNA direct targets that are experimentally verified, gene-gene interactions (edges) data were processed to perform depth-first search (DFS) algorithm, expanding all gene connections from each direct target (node) of miRNAs until no children node is found ( 21 ). For instance, Supplementary Figure S2A shows two MTIs (miR-Gene1, miR-Gene2) and five gene-gene interactions relative to a miRNA of interest. The 5 × 5 matrix in Supplementary Figure S2B was generated according to seven interactions (edges) of five elements (nodes), giving each filed value a ‘1’ if there exists the interaction between two elements, whereas a ‘0’ if no interaction. After applying DFS algorithm, an entire spanning tree was constructed ( Supplementary Figure S2C ) even if the regulation of feedback loop or self-control occurs. Through the above processes, entire miRNA indirect targets were distinguished.
For function annotation of Arabidopsis miRNA-mediated genes, the latest GO terms were collected from GO consortium ( 22 ). The hypergeometric distribution was applied to calculate the P -value of GO enrichment.
Reconstructing regulatory networks of Arabidopsis miRNAs
By combining TF-miRNA regulatory relations, putative and experimentally confirmed MTIs, and downstream genetic circuits (indirect targets), regulatory networks of Arabidopsis miRNAs were reconstructed. To visualize the inferred networks, Cytoscape, an open-source software used to illustrate biomolecular interaction networks such as protein-protein, protein-DNA and genetic interactions, was applied ( 23 ).
Results and Discussion
Arabidopsis miRNA promoters
Unlike miRNAs in animals, miRNAs in plants are primarily encoded in intergenic regions and have their own promoters. According to the previous studies, most plant miRNA genes are class II genes (transcribed by RNA polymerase II), and are regulated by similar mechanisms with protein-coding genes ( 24 ). Therefore, the strategies based on chromatin structure or sequence features for promoter prediction in coding genes are useful for identifying TSSs of plant miRNAs.
Ha etal. ( 25 ) indicated that epigenetic marks such as histone H3 lysine 4 tri-methylation (H3K4me3) and H3 lysine 9 acetylation (H3K9ac) were usually enriched around TSSs, whereas nucleosome H3 were often depleted at TSSs. Moreover, TSS tags with 5′-end of full length cDNAs are also applied to determine the 5′-boundaries of pri-miRNAs ( 26 ). Furthermore, in silico analysis revealed that several core promoter elements such as Inr, REG and CA elements were also positively correlated to TSS loci ( 27 ). Notably, TSS tags, histone modification of H3K9ac and H3K4me3, and five core promoter motifs are enriched around experimentally verified TSSs, whereas nucleosome H3 are declined ( Figure 2 ). With the application of SVM, the prediction model based on above TSS-relevant datasets was created to determine Arabidopsis miRNA TSSs. Figure 3 summarizes the workflow of the SVM model used in AtmiRNET. Because SVM is sensitive to irrelevant and redundant data, efficient and robust feature selection is the critical process to enhance classification performance ( 28 ). To acquire the optimal features for model training, how each feature contributes to the accuracy of prediction model was estimated. Totally, 22 combinations of features derived from datasets used for model training were examined, and 5-fold CV was used to evaluate the performance of each combinatorial model. The result in Supplementary Table S1 suggests that the SVM model with all of the datasets, including TSS tags, ChIP-seq for H3, H3K9ac and H3K4me3, as well as core promoter motifs, possessed the best performance (precision: 91.28%; sensitivity: 91.23%; specificity: 91.28%; accuracy: 91.25%) and was used for further prediction of Arabidopsis miRNA TSSs.
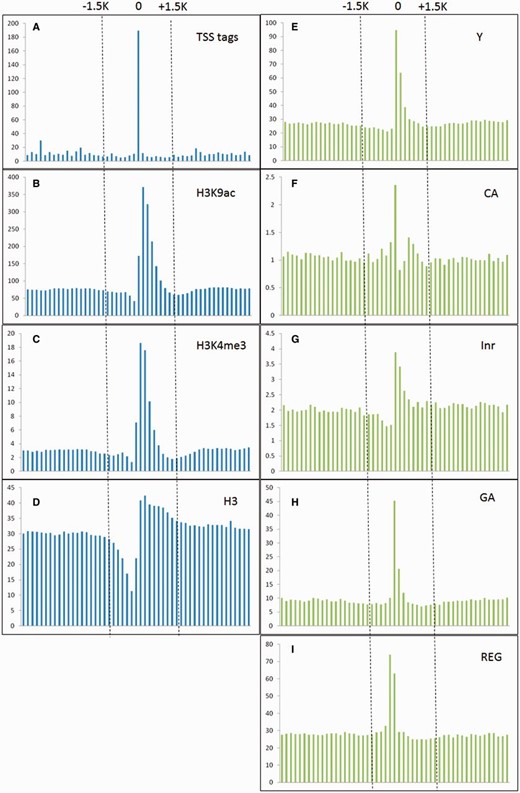
TSS tags, histone modification of H3K9ac and H3K4me3, and core promoter motifs (Y patch, CA, Inr, GA and REG) are enriched around experimentally verified TSSs, whereas nucleosome H3 are declined. The vertical and horizontal axis represent the accumulative number of each feature (X 1000 ) and genomic location (from −5 to +5 kb relative to experimentally verified TSSs, window size = 200 bp) representatively.
To our current knowledge, only a limited number of miRNA promoters have been determined in plants. Although Xie et al. ( 29 ) reported 63 TSSs for 52 Arabidopsis miRNAs by 5′-RACE, most of Arabidopsis miRNAs TSSs remain unknown. Another drawback frequently occurs when the upstream sequences of 5′-start of miRNA precursors were retrieved as miRNA promoters for further analysis in several studies ( 30 , 31 ). Rather than using ambiguous Arabidopsis miRNA promoters, the ‘Promoter’ function in AtmiRNET uses high-throughput sequencing datasets from TSS-relevant experiments of A. thaliana and plant-specific core promoter motifs enriched around TSSs to reveal the TSSs of Arabidopsis miRNAs. In total, 97% Arabidopsis miRNA TSSs (281 out of 290 miRNAs) were provided in this study. Among them, 44 miRNAs were defined as ‘intragenic’ because their pre-miRNAs embedded in the same strand of annotated genes. AtmiRNET used TSSs of host genes to represent the intragenic miRNA TSSs. By searching miRNAs of interest, users can access reliable TSSs along with the distribution of experimental evidence (an example is shown in Figure 4 ). The promoter sequence (5000-bp upstream from putative TSSs) of top 10 TSS candidates for each Arabidopsis miRNA gene can be downloaded in FASTA format.
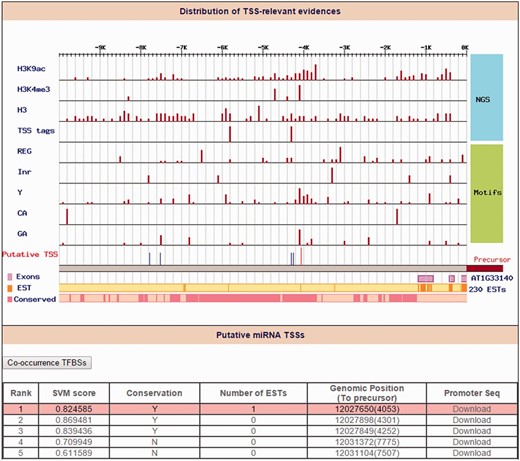
Identification of ath-miR5630b TSS. Distribution of TSS-relevant evidence (including 4 high-throughput sequencing datasets and 5 core promoter motifs) is displayed in the 10 kb upstream of the ath-miR5630b precursor. The orange line and the pink rectangles represent the initiation of expressed sequence tags (ESTs) and conservation blocks, respectively. On the other hand, the red line denotes the representative TSS for ath-miR5630b (Rank 1), and the blue lines denote other TSS candidates of ath-miR5630b.
Putative TF-miRNA relations were explored according to the occurrence of coTFBS
Sequence-based computational approaches, e.g. PWM, are widely used to search specific TF-binding motifs within promoter regions. However, short sequence motif arising unexpected false positive rate is a critical problem. Based on the hypothesis that genes with co-expression pattern may be regulated by common TFs, the concept of coTFBSs, the common (co-occurrence) TFBS motifs coincide in promoters of a miRNA and its co-expressed annotated genes, was implemented in AtmiRNET. Annotated genes coexpressed with Arabidopsis miRNAs were collected to determine coincident TFBSs in their promoters. A specific TFBS located in the promoter regions of most genes in the same coexpressed group implies its corresponding TF is the most possible one controlling the expression of miRNAs. Moreover, to ensure that the over-represented TFBSs are statistically significant rather than randomly occurred, hypergeometric P -value for each TFBS was calculated.
Consequently, users can explore TF-miRNA regulatory relations by either miRNA search (TF candidates that regulate an Arabidopsis miRNA of interest) or TF search (what Arabidopsis miRNAs are regulated by a specific TF) by using ‘Regulator’ function in AtmiRNET. We use default (PCC, raw intensity and internal control Actin) to recommend putative TFs of Arabidopsis miRNAs with the frequency of coTFBS >50% and hypergeometric P -values <0.1. However, users are allowed to customize the parameters. Then, the binding sites and sequence motifs located in promoter regions are shown interactively by the dynamic display according to selected TF candidates. Furthermore, the co-expressed gene lists of Arabidopsis miRNAs are available for reference. Figure 5 demonstrates the search result for detecting cis - and trans -elements of ath-miR167c.
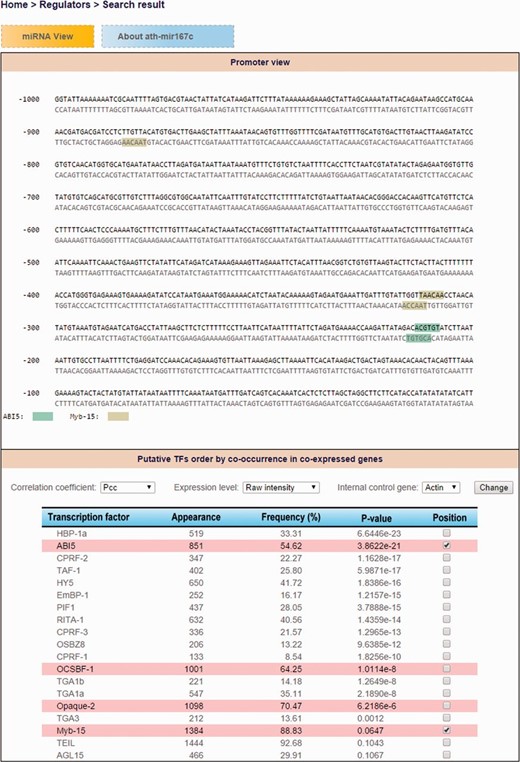
The demonstration of search result for detecting cis- and trans- elements of ath-miR167c.
Other features in AtmiRNET
Apart from providing direct and indirect targets, the correlations between the expression levels of miRNAs and their targets are depicted in AtmiRNET to evaluate the validity of target prediction. Moreover, the enriched GO terms of miRNA-mediated genes allows users to deduce biological functions of miRNAs in Arabidopsis , which are still inadequate until now. For network reconstruction, given the graphic summary of interactions pivoted around miRNAs, users gain a perspective on the biological roles of Arabidopsis miRNAs intuitively. Figure 6 illustrates an example of inferred regulatory network in Arabidopsis . Users are allowed to customize the parameters for detecting cis - and trans -elements and switch to the update networks.
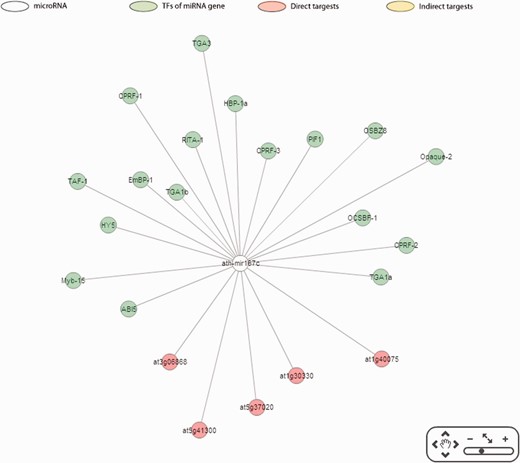
Case study
ath-miR167c may play a crucial role in the crosstalk between ABA and auxin signaling pathways
In A. thaliana , plenty of evidence shows that pathways of abscisic acid (ABA) and auxin impinge on each other, suggesting the signaling crosstalk between these two plant hormones ( 32 ). ABA is believed to be related to seed development, abiotic stresses and pathogens in plants, whereas auxin, i.e. indole-3-acetic acid, is essential for numerous aspects of plant growth and development as well as environmental stresses. Rock and Sun ( 32 ) conferred possible mechanisms of crosstalk between ABA and auxin signaling pathways in roots of Arabidopsis ; however, none of them was experimentally confirmed.
Moreover, Liu et al . ( 33 ) surveyed the effects of three abiotic stresses including high-salinity, drought and low temperature in Arabidopsis and identified 14 stress-induced miRNAs by microarray data. Among them, miR167 was induced by both high salinity and drought, and miR167c was gradually increased from 2 to 24 h after exposure to high-salinity treatment. Interestingly, the result from AtmiRNET shows that two ABA-induced TFs, ABI5 and Myb-15, regulate miR167c, and two of miR167c target genes are auxin response factor 6 (ARF6) and auxin response factor 8 (ARF8) ( Figure 7 ) ( 34 ). It reveals that miR167c may involve in stress resistance by the influence of auxin signaling pathways, and miR167c possibly connects the crosstalk between ABA and auxin signaling pathways. It is noticed that Ding et al. ( 35 ) suggested that transgenic overexpression of MYB15 confers enhanced sensitivity to ABA and improves drought and salt tolerance in Arabidopsis , which supports our finding. This important finding should be further proved.
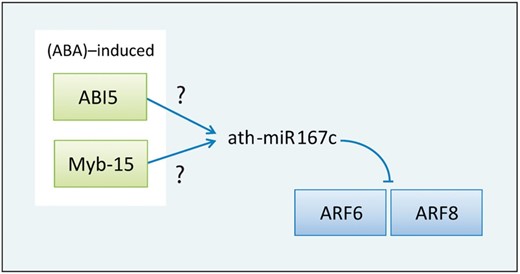
An inferred regulatory relation revolves around ath-miR167c. ABA-induced ABI5 and Myb-15 regulate miR167c, and miR167c targets ARF6 and ARF8.
Repression of ath-miR156a and miR156c triggers vegetative phase change in Arabidopsis
By using ‘Targets’ function in AtmiRNET, 13 putative targets of ath-miR156a are identified. Most of them are Squamosa promoter-binding protein-like TF family. Notably, the negative correlation of expression levels between miR156a and its targets is observed. The functional enrichment analysis of GO terms reveals that miR156a may be relative to DNA binding, development and regulation of vegetative phase change. In 2013, Yang et al. ( 36 ) indicated that vegetative phase change is initiated by a decrease in miR156, and miR156a and miR156c were found to play dominant roles in this transition. This evidence supports the inference of miR156a functions in AtmiRNET (also for miR156c). Supplementary Figure S1 depicts the regulatory network of miR156a consisted of its TFs, direct targets and indirect targets.
Conclusion
Unlike animal miRNAs, our knowledge of miRNA function is comparatively insufficient in plants. Although several of bioinformatics tools or resources are geared toward plant miRNAs, it is a challenge to understand how plant miRNAs involve in significant pathways or bioreactions without reconstructing regulatory networks. In 2012, Liu et al. ( 37 ) developed an integrated database for Arabidopsis miRNA function annotations; however, the regulation of miRNA transcription was omitted. Hence, AtmiRNET is the first resource integrating TF-miRNA relations (upstream) and MTIs (downstream) of Arabidopsis miRNA to facilitate the reconstruction of regulatory networks and function discovery. Two case studies, the involvement of miR167c in the crosstalk between ABA and auxin-signaling pathways and miR156a in vegetative phase change, are demonstrated to reveal the potency of AtmiRNET and the fulfillment of the unmet demand in plant miRNA research.
Supplementary Data
Supplementary data are available at Database Online.
Acknowledgements
The authors would like to thank the Ministry of Science and Technology and National Science Council of the Republic of China for financially supporting this research.
Funding
This work was supported by Innovative Translational Agricultural Research Grant of Academia Sinica, Ministry of Science and Technology (MOST 103-2311-B-006-001) and National Science Council (NSC 102-2313-B-006-004) of the Republic of China. Funding for open access charge: Academia Sinica (Innovative Translational Agricultural Research Grant).
Conflict of interest . None declared.
References
Author notes
† These authors contributed equally to this work.
Citation details: Chien,C.-H., Chiang-Hsieh,Y.-F., Chen, Y.-A., et al. AtmiRNET: a web-based resource for reconstructing regulatory networks of Arabidopsis microRNAs. Database (2015) Vol. 2015: article ID bav042; doi:10.1093/database/bav042



