-
PDF
- Split View
-
Views
-
Cite
Cite
Jin-liang Zhu, Hong-Jian Gao, Li-hua Wang, Zhi-tao Yin, Mucinous adenocarcinoma of the rectum masquerading as inflammatory bowel disease—a case report, Journal of Surgical Case Reports, Volume 2025, Issue 9, September 2025, rjaf762, https://doi.org/10.1093/jscr/rjaf762
Close - Share Icon Share
Abstract
The diagnosis of mucinous adenocarcinoma (MAC) is often challenging, particularly when it is confused with other entities such as inflammatory bowel disease (IBD) or benign pathologies. In this case, a 69-year-old woman presented with chronic constipation and was initially diagnosed with IBD after multiple institutions being consulted for her chronic symptoms. After experimental treatment with mesalazine for 6 months without improvement, she sought further consultation at our hospital. In cases of smooth surfaced mass with circumscribed projections in the rectal lumen, MAC should be considered as a potential diagnosis. To differentiate this entity from other pathologic entities, high sensitivity analysis is necessary for accurate classification. In ambiguous histopathological findings, implementing multi-point sampling strategies combined with targeted deep sampling at critical anatomical landmarks can significantly enhance diagnostic accuracy and facilitate a more comprehensive pathobiological evaluation of the disease.
Highlights:
1) We identified a distinct presentation of rectal mucinous adenocarcinoma.
2) When evaluating rectal masses resembling inflammatory bowel disease with inconclusive histopathological findings, in addition to employing the conventional multi-point sampling approach, targeted deep biopsies at critical anatomical landmarks can substantially enhance diagnostic accuracy.
Introduction
Mucinous adenocarcinoma (MAC) of the rectum is a relatively rare histologic subtype of colorectal cancer (CRC), representing 5%–20% of rectal cancers, and is characterized by the abundant production of extracellular mucin, comprising over 50% of the tumor volume [1, 2]. In comparison to non-NMAC, MAC exhibits distinct characteristics in terms of clinical features, molecular properties, and pathological findings. Furthermore, MAC, with a higher incidence in female patients, is often diagnosed at advanced stages and typically shows limited efficacy in response to chemotherapy [3–6]. This type of cancer can be challenging in differential diagnosis as it may simulate other, less dangerous, lesions. The differential diagnosis must be made with benign or inflammatory pathologic proces as inflammatory bowel disease (IBD). Here, we report a case of MAC of the rectum that was almost misdiagnosed and treated as IBD.
The work has been reported in line with the SCARE criteria [7].
Case report
A 69-year-old female patient was discovered to have more frequent bowel movements without obvious cause 6 months ago, with 3–5 defecations per day. She also complained that she has endured increasingly severe constipation symptoms in recent month, accompanied by a sense of urgency and tenesmus, narrowing of stool, inability to form solid stools, and occasional abdominal pain during defecation, but no obvious bloody stool. Therefore, the patient sought treatment at multiple hospitals’ colorectal departments and underwent several colonoscopies and pathological examinations. The colonoscopy reveals that about 0.5 cm above the anal dentate line, a two-thirds circumferential hyperplastic alteration is distinguishable, with a longitudinal length of about 2.0 cm. The surface is smooth, accompanied by localized erythema and nodularity, and the lumen is constricted at the lesion site. Biopsy was taken (Fig. 1). The pathological diagnoses in all instances indicated chronic inflammation of the rectal mucosa along with superficial erosion of the mucosa. The physician has tentatively diagnosed the patient with IBD and prescribed oral and rectal medications, such as mesalazine, for treatment. However, the response to the medication has been limited. For further diagnosis and treatment, the patient was admitted to our hospital.The patient had no fever, nausea and vomiting, normal diet and sleep, weight loss of about 5 kg, and normal urination. The patient has the medical history of surgery for the removal of uterine and ovarian cysts 10 years ago. There are no other medical histories or medication allergies. She has no history of smoking, alcohol intake, or drug abuse.
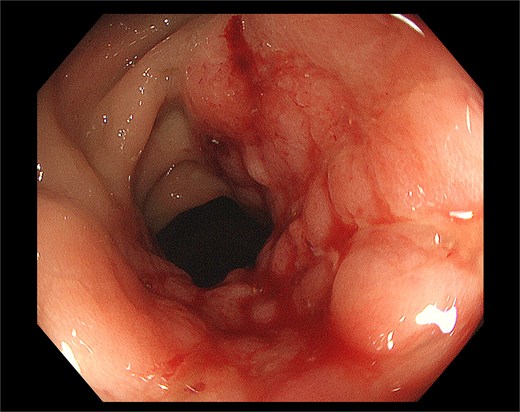
Pre-operative colonoscopy identified a smoothly surfaced mass with nodular protrusions in the rectal mucosa, exhibiting features reminiscent of inflammatory bowel disease (IBD), prompting subsequent local biopsy.
The relevant test outcomes are presented as follows:
Digital rectal examination: During the digital rectal examination, the probe reached 5 cm into the anus. A hard mass encircling approximately two-third of the rectal circumference was palpable. The surface was smooth, presenting a cobblestone-like protuberant tumor with limited mobility. No blood staining was observed on the finger cot upon withdrawal.
Rectal ultrasound: An endoscopic transrectal ultrasound probe was utilized to examine the rectum, disclosing a heterogeneous hypoechoic solid mass in the anterior and left walls of the rectum, accounting for approximately three-fourth of the circumference of the rectum. The upper margin of the lesion was 8 cm from the anal margin, and the lower margin was 4 cm from the anal margin. The boundary was ill-defined, the shape was irregular, and the lesion protruded into the lumen of the rectum, with a broad base located in the muscular layer and the surface resembling cobblestones. The lesion exhibited limited mobility, and the surrounding colonic wall layers were clearly distinguishable. Color Doppler flow Imaging detected abundant color flow.
Pelvic computed tomography (CT): The bladder is filled to an adequate extent, and its wall is thin and homogeneous. The uterine area is relatively small. A lesion ~4.8 × 3.6 cm in dimension is discerned in the right adnexal area, characterized by uniform density and a CT value of ~19 hu. The rectal mucosa is thickened, suspected to represent a lesion, and strand-like and nodular shadows are detectable in the surrounding region.
The assessment of tumor markers demonstrated that the serum level of CEA was 11.1 ng/ml, exceeding the normal upper limit by more than 1.1 ng/ml. AFP, CA153, CA199, CA50, and CA242 remained within the normal range.
Previously, all pathological biopsies were conducted using single-use endoscopic colon biopsy forceps with a tip diameter of 2.3 mm and an opening angle of 87°. These physical constraints limited the amount of tissue collected per examination due to the small size of the biopsy forceps and the narrow opening angle. To address this limitation and improve diagnostic accuracy, we adopted the transanal endoscopic microscopy (TEM) method for tissue analysis during the biopsy procedure. This advanced imaging technique enabled us to obtain larger, more representative samples through a multi-point sampling strategy supplemented by targeted deep sampling at critical anatomical landmarks. Consequently, the increased sample size and improved tissue representation enhanced the diagnostic capabilities of the analysis. Pathological examination revealed the presence of high-grade intraepithelial neoplasia and dysplasia, demonstrating the effectiveness of the novel approach in improving diagnostic accuracy.
Due to the fact that the tumor is merely 3 cm away from the anal margin, if the surgery for malignant tumor is carried out, the patient will lose the function of the anus and have a permanent colostomy on the abdominal wall. A multidisciplinary team comprising oncologists, gastrointestinal surgical specialists, gastroenterologists, ultrasonographer, radiologist, and pathologists constituted to further deliberate and elucidate the treatment plan for the disease. Following consultation with numerous experts, the clinical diagnosis still deems the possibility of the patient having a malignant tumor to be relatively high. A second preoperative biopsy can be conducted to confirm the diagnosis. Alternatively, a Miles procedure can be carried out directly, but this demands the consent of the patient and her family. After consulting with the patient and her family, the patient and her family requested a direct Miles procedure, and the informed consent form was signed. The postoperative pathology disclosed that the tumor was a MAC of the rectum, invading the serosa, with no vascular or neural invasion noted. The budding grade was low. No metastases were detected in the first stationary lymph node (N1: 0/17); no metastases were detected in the second stationary lymph node (N2: 0/6); no metastases were detected in the third stationary lymph node (N1: 0/2); in total (0/25). The pathological staging was pT3N0Mx (AJCC 2017, 8th edn). A standout characteristic of this specimen is the precise localization of the tumor within the submucosal layer. The tumor is located at a distance of ~1905.26 um from the interface between the normal mucosal layer and the submucosal layer, where it originates. Simultaneously, the underlying mucosal architecture remains structurally intact, free from detectable signs of pathological change (Fig. 2).
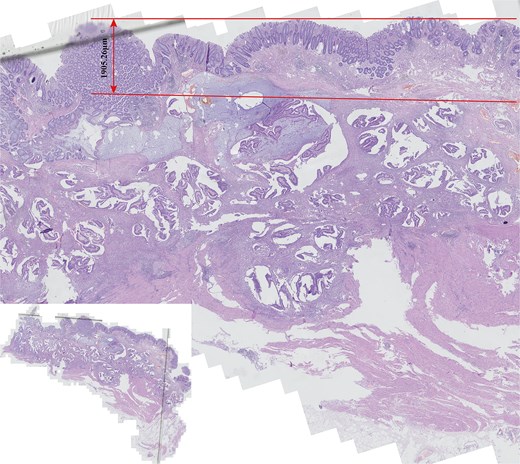
Comprehensive histological examination of the entire postoperative pathological specimen revealed that rectal mucinous adenocarcinoma (MAC) exhibited near-normal histological characteristics in the mucosal layer, with strict localization within the submucosa (the small image located in the lower left corner provides a panoramic view of the entire tissue section).
Discussion
The majority of clinical studies have affirmed that the prognosis of rectal MAC is unsatisfactory [2, 6, 8, 9]. Consequently, early diagnosis and treatment are of utmost importance. However, rectal MAC can sometimes emerge stealthily [10]. Its clinical manifestations closely mimic those of IBD, and the lesion often resides in the submucosa, making the differential diagnosis between MAC and IBD exceptionally difficult. The pathological diagnosis forceps used in conventional colonoscopic sampling typically have a maximum insertion depth of ~2 mm. This limitation becomes particularly apparent when encountering tumors located within the submucosal layer, as the restricted access to this deeper anatomical layer often results in insufficient tissue sample size, thereby complicating accurate diagnoses. The application of TEM in pathological biopsies may provide a valuable and effective complement for cases that present diagnostic challenges.
IBD mainly comprises the Crohn’s disease (CD), and ulcerative colitis (UC). The two diseases vary in their primary sites of occurrence, the depth of invasion into the intestinal wall, and the mode of dissemination [11]. CD induces transmural ulceration affecting any segment of the gastrointestinal tract. It predominantly affects the terminal ileum or the perianal region in a non-continuous mode. The clinical manifestations of CD mainly consist of recurrent abdominal pain, watery diarrhea, and weight loss. UC is characterized by diffuse inflammation that is confined to the colon. It typically commences in the rectum, spreads proximally in a continuous manner and often encompasses the entire colon. The interval from the manifestation of symptoms to diagnosis is typically shorter in UC than in CD, usually transpiring within a few weeks to several months, whereas CD could potentially take several years. The severity of symptoms is correlated to the extent of inflammation. Commonly, symptoms encompass diarrhea, abdominal pain, an increased frequency of bowel movements (rectal inflammation), tenesmus, accompanied by or without mucus and bloody stool [12].
Currently, there is no definitive diagnostic criterion for IBD. Diagnosis typically relies on a combination of clinical observations, endoscopic findings, histopathological examination, laboratory assays, and imaging studies. These clinical features of IBD are often utilized as differential diagnostic considerations for MAC, particularly in cases where other pathological entities may present with similar symptoms or imaging abnormalities. However, some clinical manifestations of IBD may lack the distinctiveness previously noted, necessitating careful correlation across multiple diagnostic modalities to confirm a diagnosis.
IBD is also a significant risk factor for CRC. For patients with persistent active diseases and abnormal colonic mucosa, the effect of simple colonoscopy surveillance is unsatisfactory, and colorectal resection surgery still requires the support of pathological diagnosis. Choi and colleagues reviewed a 40-year surveillance program in the UK and discovered that among patients with high-grade dysplasia (HGD) detected by colonoscopy, 55.2% were found to have occult cancer in their resected colonic specimens. The high incidence of occult cancer clearly demonstrates the necessity of colorectal resection when HGD is present [13]. Cannon provided a treatment plan for dysplasia detected by endoscopy, stating that the presence of HGD in apparently normal mucosa is an indication for proctocolectomy [14]. Bernstein et al. also suggest that immediate colectomy is essential for all patients diagnosed with high-grade or low-grade dysplasia [15]. These studies above indicate that surgical treatment should be regarded as the main treatment option, even if the pathological diagnosis is high-grade or low-grade intraepithelial neoplasia rather than cancer.
Therefore, when the clinical symptoms are similar and rectal lesions present a ring-shaped constriction, it should be given serious attention, and a strategy of multi-point collection and deep sampling in pathological biopsy should be employed. During the conventional pathological biopsy process using forceps, insufficient mechanical transmission efficiency and limitations in clamping surface design significantly reduce the success rate of obtaining samples from deep lesion areas. This case highlights the diagnostic challenges in MAC of the rectum, particularly when symptoms mimic other gastrointestinal diseases such as IBD.
MAC of the rectum may present with symptoms resembling those of IBD, resulting in delayed diagnosis and potentially severe complications if misdiagnosed. Clinicians should maintain a high index of suspicion when evaluating patients with IBD-like symptoms, especially when imaging studies fail to confirm a definitive tumor but reveal suspicious findings. In these cases, TEM biopsy or endoscopic ultrasound-guided fine-needle aspiration biopsy can be considered as complementary tools for histopathological assessment, provided the suspected lesion does not extensively invade the intestinal mucosa. Early and accurate diagnosis is crucial for optimizing patient outcomes through appropriate therapeutic strategies.
Author contributions
Z.Y., J.Z., and H.G. contributed to the conception and study planning. J.Z. wrote the first draft of the manuscript. J.Z. and L.W. prepared Figs 1 and 2. J.Z., L.W., H.G., and Z. Y. revised the manuscript. All authors reviewed and approved the final manuscript.
Conflict of interest statement
None declared.
Funding
The authors received no specific funding for this study.
Data availability
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.
Study approval statement
No ethics approval was required for the publishing of this case report. This retrospective review of patient data did not require ethical approval in accordance with local/ national guidelines.
Consent
Written informed consent was obtained from the patient for publication of this case report. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.



