-
PDF
- Split View
-
Views
-
Cite
Cite
Hui Wen Chua, Jabed Iqbal, Lianne A L Lee, Lymphoma in the breast: a diagnostic and treatment dilemma, Journal of Surgical Case Reports, Volume 2025, Issue 9, September 2025, rjaf698, https://doi.org/10.1093/jscr/rjaf698
Close - Share Icon Share
Abstract
Lymphoma involving the breast is an uncommon clinical entity, comprising ˂0.5% of all breast malignancies. Due to its rarity, there is no standardized treatment approach. We describe three patients managed at our centre, each representing a distinct presentation of breast lymphoma: primary breast lymphoma, secondary breast lymphoma, and synchronous disease with concurrent invasive breast carcinoma and lymphoma. Management varied based on histological subtype and disease extent. Therapeutic strategies included combinations of surgery, systemic chemotherapy, and radiotherapy. Each case was discussed in a multidisciplinary tumour board to guide individualized treatment. Breast lymphoma presents diagnostic and therapeutic challenges. Management with multidisciplinary input should be tailored according to lymphoma subtype and patient-specific factors. Further studies are warranted to establish evidence-based treatment protocols.
Introduction
Lymphoma involving the breast is rare, representing < 0.5% of all malignant breast neoplasms, due to the paucity of lymphoid tissue within the breast [1]. Primary breast lymphoma (PBL), as defined by Wiseman and Liao, must fulfil the criteria: (1) adequate material for diagnosis; (2) proximity of mammary tissue and lymphomatous infiltrates; (3) absence of disseminated disease; and (4) no prior history of extramammary lymphoma [2]. PBL typically involves the breast and ipsilateral axillary nodes, accounting for 0.85%–2.2% of extra-nodal malignant lymphomas. In contrast, secondary breast lymphoma (SBL), representing system lymphoma with secondary breast involvement, is more frequently encountered [3]. A third, rarer category is synchronous disease, where breast carcinoma and lymphoma co-exist.
We conducted a retrospective review of patients diagnosed with breast lymphoma at our breast centre.
Between 2018 and 2021, 1455 patients were treated for breast cancer at our centre. Three female patients with diagnosed. (0.2% of all cases) None exhibited B symptoms (fevers, night sweats, loss of weight), or had prior history of lymphoma and breast implants. They underwent triple assessment with diagnostic breast imaging and biopsy for definitive diagnosis. They were diagnosed in 2018 and 2019 and remain under active surveillance.
Clinical features and treatment
Case 1 (Primary breast lymphoma)
Patient A is a 60-year-old lady with diabetes. She presented with 2 weeks of right nipple pruritus and bloody nipple discharge. Examination showed dry skin over the nipple areolar complex and bloody nipple discharge on expression. On imaging, there was a 12 mm mass with dilated debris-filled duct, and an enlarged ipsilateral axillary lymph node (Figs 1 and 2). Core needle biopsy of the breast and node showed triple negative invasive breast carcinoma together with marginal zone B-cell lymphoma (MALT-type) (Fig. 3). A positron emission tomography (PET) scan showed no evidence of additional hypermetabolic activity. She underwent mastectomy, having opted against breast conserving surgery, followed by adjuvant chemotherapy with doxorubicin, cyclophosphamide, and Paclitaxel (AC-T regimen) for triple negative breast cancer. As the MALT lymphoma was localized and indolent, no additional adjuvant treatment was required.
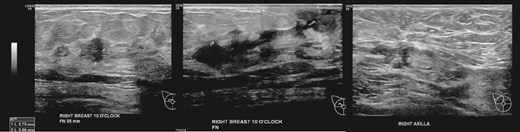
(Left, middle) Ultrasound images of the right breast irregular spiculated hypoechoic mass with ill-defined margins, associated with a dilated duct 3 mm; (Right) Ultrasound image of the enlarged axillary lymph node.
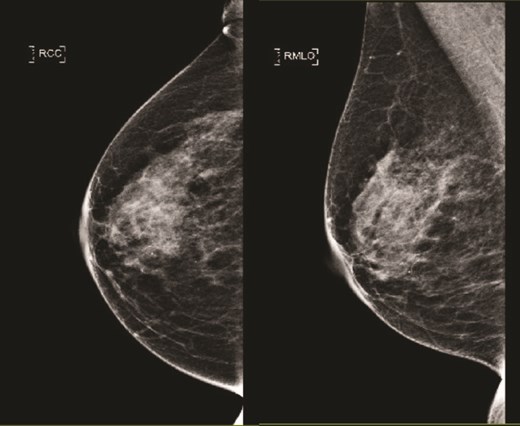
(Left) Mammography cranial-caudal view of right breast; (Right) Mammography mediolateral oblique view of the right breast: Both showing few loose clusters of faint and punctate microcalcifications in the upper outer right breast with no suspicious linear or tight cluster of microcalcifications.
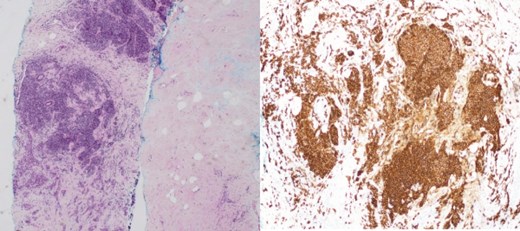
(Left) Breast core biopsy showing periductal atypical lymphoid proliferation. (H&E stain, 20 times magnification); (Right) Breast core biopsy with immunohistochemical staining showing atypical lymphoid cells positive for CD20 stains.
Case 2 (Secondary breast lymphoma)
Patient B, aged 72, was healthy and presented with left breast pain and a breast lump for 5 months. A 15 mm left breast lump was palpable. Imaging confirmed this finding and revealed bilateral axillary lymphadenopathy (Figs 4 and 5). Core biopsies of the left breast nodule and bilateral axillary nodes were diagnostic of chronic B cell lymphocytic lymphoma (Fig. 6). Positron emission tomography (PET) scan demonstrated disseminated lymphadenopathy involving cervical, mediastinum, and intra-abdominal lymph nodes. As the disease was indolent and she was asymptomatic, she was conservatively managed with active surveillance. Her breast discomfort resolved with analgesia. Follow-up imaging demonstrated stable lymphadenopathy.
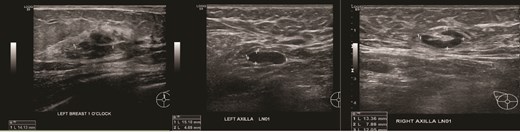
(Left) Ultrasound images of the left breast ill-defined hypoechoic mass; (Middle, Right) Ultrasound images of the axillary lymph nodes with cortical thickening and effacement of the fatty hilum.
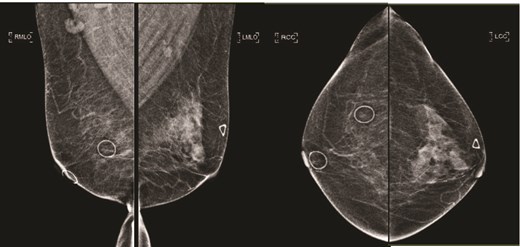
(Left) Mammography medioloateral oblique views of bilateral breasts; (Right) Mammography cranial-caudal views of bilateral breasts: Left upper central breast shows a vague focal asymmetry corresponding to the palpable concern, and several prominent right axillary lymph nodes.
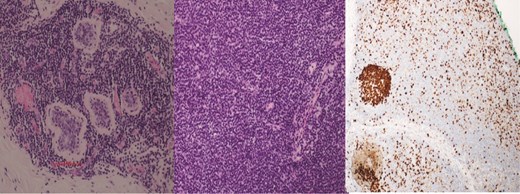
(Left) Breast core biopsy showing neoplastic lymphoproliferation around benign duct (H&E stain, 20 times magnification); (Middle) Breast core biopsy with immunohistochemical staining showing atypical lymphoid cells positive for CD20 stains; (Right) lymph node core biopsy showing distorted architecture by an infiltrate of small lymphocytes involving entire node (H&E stain, 20 times magnification).
Case 3 (Synchronous disease)
Patient C, aged 70, had no significant past medical history and initially presented to Otolaryngology with right-sided cervical lymphadenopathy. Excision biopsy confirmed a diagnosis of diffuse large B cell lymphoma (DLBCL). She then reported a 3-month history of a left breast lump. Clinical examination and imaging revealed a 20 mm lesion associated with ipsilateral axillary lymphadenopathy (Figs 7 and 8). Triple assessment confirmed triple negative invasive ductal carcinoma in the breast, whilst there were follicular cells in the nodes (Fig. 9). She underwent systemic chemotherapy for stage III diffuse large B-cell lymphoma using Rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone (R-CHOP) protocol followed by weekly Paclitaxel for the concurrent breast cancer. She achieved clinical remission for lymphoma. Subsequently she underwent breast conserving surgery with sentinel node biopsy, and adjuvant chest radiotherapy.
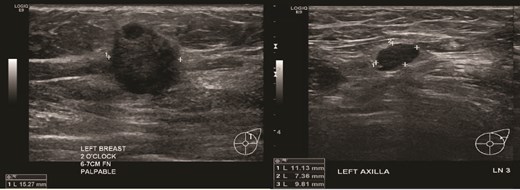
(Left) Ultrasound image of the left breast hypoechoic lesion; (Right) Ultrasound image of one of several enlarged lymph nodes with effacement of fatty hilum.
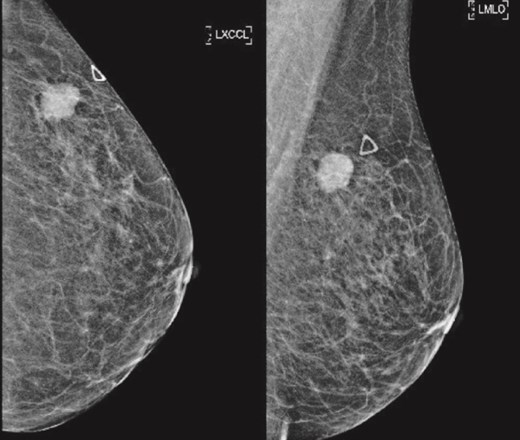
(Left) Mammography cranial-caudal view of left breast; (Right) Mammography mediolateral oblique view of the left breast: Left upper outer breast spiculated mass with minimal architectural distortion.
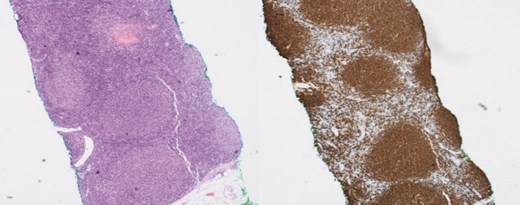
(Left) Breast core biopsy showing follicular hyperplastic nodules of atypical lymphoid cells (H&E stain, 40 times magnification); (Right) Breast core biopsy with immunohistochemical staining; atypical lymphoid cells forming hyperplastic nodules positive for CD20 stains.
Discussion
The lifetime risk of developing non-Hodgkin lymphoma is estimated at 1.8%, and PBL accounts for only ⁓2% of all extranodal lymphomas [4, 5]. In this paper, we highlight three distinct clinical scenarios involving the breast: PBL (Patient A), SBL (Patient B), and synchronous disease (Patient C). These cases illustrate the variability in presentation, management, and clinical outcomes of breast lymphoma.
PBL typically presents in women aged 60–65 years, slightly younger than SBL, which is between 60–70 years [6–8]. A painless palpable mass is the most commonly reported presenting symptom, but a milieu of symptoms exist, such as skin retraction and erythema in those with diffuse parenchymal involvement. Up to 10% of breast lymphomas are subclinical and only detected on screening mammography. B symptoms are more frequently observed in SBL than PBL due to the presence of extramammary lymphoma. In patient A, the MALT lymphoma was likely asymptomatic and discovered incidentally with the symptomatic invasive ductal carcinoma, which presented with bloody nipple discharge.
DLBCL is the most prevalent histologic subtype of PBL, comprising 45%–79% of cases, followed by follicular lymphoma (15%), MALT lymphoma (12.2%), and Burkitt lymphoma (10.3%) [7–9]. SBL, most commonly presents as DLBCL, with follicular and MALT subtypes being less frequent [10]. Bilateral axillary lymphadenopathy on imaging may be suggestive of SBL and histological confirmation via biopsy is recommended for diagnosis.
Due to the rarity of both PBL and SBL, there is no established consensus on the optimal treatment strategy, with most evidence from small retrospective studies and case reports. Wong et al. (Mayo Clinic) advocate for a multi-modal approach, tailoring treatment to histological subtype [5]. Anthracycline-based chemotherapy is typically administered for high-grade lymphomas, whilst radiation alone may suffice for indolent forms. Notably, mastectomy does not confer survival or recurrence benefit and is not routinely recommended. Breast conserving surgery followed by radiotherapy may be appropriate in selected cases [11, 12].
Here, Patient A received systemic chemotherapy for triple negative breast cancer, whilst the MALT lymphoma required no further intervention. Patient B was managed conservatively with surveillance for indolent systemic lymphoma. Patient C received systemic R-CHOP chemotherapy for DLBCL, followed by breast conserving therapy for invasive ductal carcinoma, benefitting from the overlap in chemotherapeutic regimens for both lymphoma and breast cancer.
These cases underscore the diagnostic and therapeutic complexity of breast lymphoma. Multi-disciplinary collaboration is essential in creating individualized treatment plans addressing both pathologies. Given the limited literature, further studies are warranted to establish evidence-based management protocols for this rare clinical scenario.
Conflict of interest statement
No conflict of interest to declare.
Funding
No sources of funding applicable.



