-
PDF
- Split View
-
Views
-
Cite
Cite
Jun Kiat Thaddaeus Tan, Madeline Yen Min Chee, Shihleone Loong, Meihuan Chang, Anal metastases from colorectal cancer – a case report and review of literature, Journal of Surgical Case Reports, Volume 2025, Issue 9, September 2025, rjaf578, https://doi.org/10.1093/jscr/rjaf578
Close - Share Icon Share
Abstract
Colorectal cancer occasionally metastasizes to the anal canal. Studies on the matter are dated and it is timely to review the current evidence. We report a case of a 52-year-old male with rectosigmoid adenocarcinoma and a metastatic anal nodule at the scar of a previously treated perianal abscess. Comparison of histological features and mutational profiling on next-generation sequencing (NGS) determined the anal lesion as metastatic adenocarcinoma arising from the rectosigmoid primary. Differentiating anal implantation metastases from a synchronous primary anal adenocarcinoma has therapeutic implications. CK7, CK20 and CDX2 immunohistochemistry can be helpful, although these have limitations in differentiating certain cell lines. In this case, NGS supplemented standard immunohistochemistry and facilitated the diagnosis of anal metastases. This case highlights unique considerations in diagnostic evaluation and management of anal implantation metastases, and the utility of NGS in determining the pattern of spread in metastatic colorectal cancer.
Introduction
Metastatic spread of colorectal cancer is guided by disease biology [1] beginning with lymphatic involvement followed by hematogenous spread [2]. Anal implantation metastases represent a unique manner of presentation [3, 4] for which studies are dated, and it is timely to review current evidence on the subject. We present a rare case of a patient with a metastatic anal nodule from an obstructing rectosigmoid primary adenocarcinoma and performed a review of current literature.
Case presentation
We present a 52-year-old male with hypertension, diabetes mellitus, dilated cardiomyopathy and a previous right-sided perianal abscess for which he underwent incision and drainage 14 years prior to admission. He had no previous colonoscopy. He presented with a 2-month history of per-rectal bleeding, constitutional symptoms and was obstipated for 2 days. On examination he had a distended abdomen but no peritonism or tenderness. A 1.5 cm irregular nodule was found at the 7 o’clock position on the anal verge, corresponding to the area of previous surgery (Fig. 1).
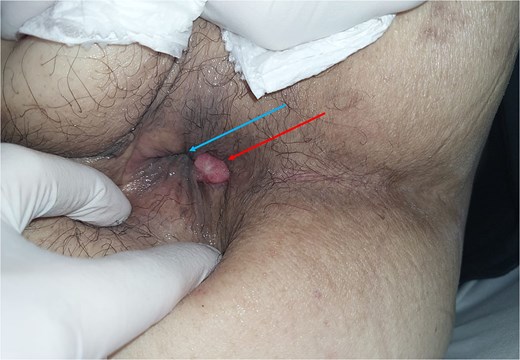
Preoperative clinical photograph of perianal exam. Anal nodule demonstrated at 7 o’clock position. Blue arrow – Anal verge. Red arrow – Anal nodule
A contrasted computed tomography (CT) scan of the thorax, abdomen and pelvis demonstrated an obstructing 8.6 × 5.3 cm rectosigmoid colonic mass with 2.4 × 2.1 cm extraserosal extension. Extensive peritoneal nodules measuring up to 6.1 × 3.4 cm, multiple hypo-enhancing bilobar hepatic nodules up to 2.4 cm (Fig. 2A) and bilateral pulmonary nodules measuring up to 0.7 cm were reported as suspicious for metastases. He had a hemoglobin count of 9.4 g/dL, alkaline phosphatase of 208 U/L and carcinoembryonic antigen (CEA) of 140 ug/L.
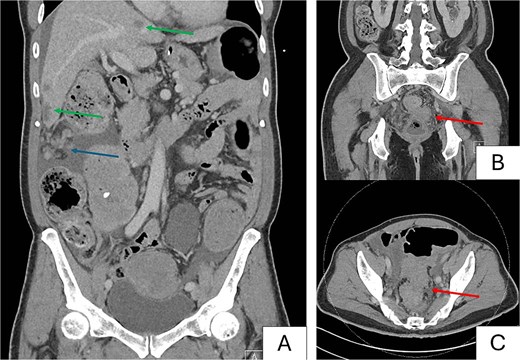
Preoperative computed tomography images. Images demonstrate a metastatic rectosigmoid mass with suspicious nodules in liver and peritoneum with features of large bowel obstruction. (A) Coronal image demonstrating bulky peritoneal nodules (blue arrow), and bilobar hepatic nodules suspicious for metastases (green arrows). (B) Coronal image demonstrating primary rectosigmoid tumor (red arrow). (C) Axial image demonstrating tumor with pericolonic nodules suspicious for nodal involvement (red arrow).
He underwent diagnostic laparoscopy, colostomy creation and flexible sigmoidoscopy. An obstructing rectosigmoid tumor 15 cm from anal verge was biopsied. The anal mass arose from the perianal skin and extended into the intersphinteric space, and an incisional biopsy was performed. Diagnostic laparoscopy revealed extensive peritoneal and omental metastases with a peritoneal carcinomatosis index (PCI) score of 11. The largest omental nodule was biopsied. A transverse colostomy was performed using two 5 mm working ports to the left flank and right hypochondrium and matured over the right hypochondrium using vicryl 3/0. Colostomy started functioning by postoperative day 1 (POD1). He tolerated full diet by POD5 and was discharged.
Histology of the rectosigmoid tumour confirmed moderately differentiated invasive adenocarcinoma, with normal MLH-1, MSH-2, MSH-6 and PMS-2 expression on routine immunohistochemistry. Specimens from the anal nodule had infiltrates of malignant glands in squamous mucosa consistent with adenocarcinoma, whereas the omental nodule demonstrated fibroadipose tissue with infiltrates of adenocarcinoma. Morphologic characteristics of all three lesions were similar. Adenocarcinoma was diffusely positive for CDX2 consistent with metastases from a colorectal primary (Fig. 3A).
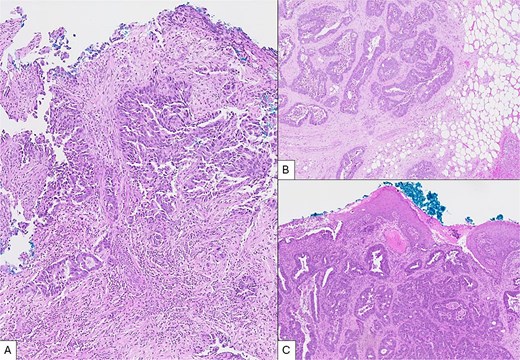
Microscopic views of intraoperative specimens (H&E, × 50). (A) Specimen from sigmoid tumour showing colonic tissue fragment with moderately differentiated invasive adenocarcinoma. (B) Tissue from omental nodule showing fibroadipose tissue with infiltrates of adenocarcinoma positive for CDX2. (C) Tissue from anal nodule with focal squamous epithelium showing infiltrates of adenocarcinoma with morphology comparable to specimens A and B.
Mutational analysis with a next generation sequencing (NGS) assay comprising a panel of 17 genes (somatic solid tumour panel) was performed on specimens from the anal and omental nodules, which discovered a similar mutational profile in both specimens including wild-type KRAS, NRAS, BRAF, and TP53 mutation (TP53 NM_000546.5, Exon10, c.1024C>T p.(Arg342*)) suggesting both specimens were of identical lineage. Taking into consideration the overall clinical picture, comparable histological characteristics between the three samples obtained, as well as supportive features on NGS, overall findings were consistent with metastatic anal and omental nodules from the rectosigmoid primary.
The patient was reviewed two weeks postoperatively with good recovery and was advised to start palliative systemic therapy. First cycle with oxaliplatin 130 mg, bolus 5-fluorouracil (5-FU) 600 mg and infusional 5-FU 3800 mg was started. He was seen well at five weeks after surgery.
Discussion
This phenomenon was first reported by Killingback et al. [4], who described four patients with colorectal cancer and anal metastases in the 1960s. All four presented with symptomatic perianal nodules found to harbor adenocarcinoma, arising from a colorectal primary. Few subsequent studies have explored the pathophysiology of this phenomenon. Nonetheless, it is well-established that primary colonic malignancies exfoliate tumor cells that have seeding potential. Some studies demonstrated tumor cells in samples retrieved from rectal washouts during colon cancer surgery [5, 6], which can seed and proliferate distally with scarred mucosa as a suitable interstitial milieu [7, 8], evident in the fact that most published literature on anal metastases involves a scarred mucosa. Two of four patients described by Killingback had a prior history of anal instrumentation [4], and others have reported metastatic involvement of fistula-in-ano from a rectal primary [3, 9, 10], and metastatic involvement after haemorrhoid surgery [11].
One diagnostic challenge is in differentiating metastases from a synchronous primary anal adenocarcinoma. Some studies suggest an approach based on clinical history alone [11]. Others correlate gross histology of both lesions to establish a diagnosis [4, 9]. Next generation sequencing (NGS) permits direct comparison of the mutational profile of both lesions to prove an association by lineage, and to our knowledge this is the first case reported in literature to use NGS in this unique context. Immunohistochemistry using CK7 and CK20 can also be useful. CK20 is a major cellular protein produced by mature enterocytes and goblet cells and are expressed in tumour cells derived from a gastrointestinal epithelial origin [12]. Ramalingam et al. found that cell lines of anal glandular origin were typically immunoreactive to CK7 but not to CK20, as compared to rectal adenocarcinoma which tends to display immunoreactivity to CK20 [13]. However, these stains will not be helpful in differentiating an intestinal-type anal adenocarcinoma from colorectal adenocarcinoma. Tissue samples from this case stained positive for CDX2, a homeobox gene encoding an intestine-specific transcription factor expressed by epithelial cells within the gastrointestinal tract. This is uniformly expressed in 76%–100% of tumor cells arising from colorectal tumors, and also mucinous ovarian and urothelial adenocarcinomas [14].
Distinguishing synchronous anal adenocarcinoma and metastatic anal involvement has therapeutic implications. Primary anal canal adenocarcinoma, given their low position and high likelihood of sphincter involvement, typically warrants consideration of neoadjuvant chemoradiotherapy potentially followed by an abdominoperineal resection, with local excision demonstrating poorer overall survival compared to radical surgery [15]. Conversely, local excision has been proposed as sufficient for curative treatment of anal metastatic nodules [9, 10], although there remains a paucity of data on long term outcomes.
Conclusion
We present an uncommon case of metastatic rectosigmoid adenocarcinoma presenting with anal metastases. NGS supplements conventional histology to elucidate cellular lineage which is critical for treatment planning. Further studies into the pathophysiology and natural history of implantation metastases would be useful to guide management.
Conflict of interest statement
The authors affirm that neither submitted material nor portions thereof have been published previously or are under consideration for publication elsewhere. There are no potential conflicts of interest to declare.
Funding
None declared.



