-
PDF
- Split View
-
Views
-
Cite
Cite
Rafif E Mattar, Osama Almubadel, Areej Bokhari, Warthin-like variant of papillary thyroid carcinoma: a rare case report, Journal of Surgical Case Reports, Volume 2025, Issue 8, August 2025, rjaf640, https://doi.org/10.1093/jscr/rjaf640
Close - Share Icon Share
Abstract
Warthin-like variant of papillary thyroid carcinoma (WL-PTC) is an extremely rare subtype of PTC characterized by oncocytic cells with a dense lymphocytic infiltrate. Despite its rarity, WL-PTC is generally associated with a favorable prognosis. We report a case of a 36-year-old gentleman who developed WL-PTC without family history or risk factors for cancer.
Introduction
Papillary thyroid carcinoma (PTC) is the most common type of thyroid cancer; it constitutes ⁓89.4% of all thyroid cancers. PTC has a good prognosis and outcomes, with a 97% in overall survival rates for 10 years [1–3]. PTC can classify based on its histological features; the Warthin-like variant of papillary thyroid carcinoma (WL-PTC) is considered an extremely rare type of PTC, where it constitutes ⁓0.2%–1.9% of PTC cases [4–6]. It is characterized by the presence of papillary structures, surrounded by oncocytic and lymphocytic cells. This tumor expresses rearranged during transfection/papillary thyroid carcinoma (RET/PTC) fusion gene [7]. WL-PTC is associated with good prognosis [5]. This case report aims to highlight the diagnostic and therapeutic challenges of WL-PTC and its clinical implications.
Case presentation
A 36-year-old gentleman presented to our clinic with a 1-month history of neck swelling, without any other complaints. The patient was medically and surgically free, had no family history or risk factors for thyroid cancer. Thyroid function tests revealed a normal thyroid-stimulating hormone (TSH) of 2.05 μIU/mL, which was within the normal range. The patient’s remaining laboratory results showed an elevated free thyroxine (T4) level of 26.7 pmol/L (reference range: 10.3–24.5 pmol/L). Corrected calcium was low at 2.03 mmol/L (reference range: 2.10–2.55 mmol/L), while magnesium, vitamin D, and albumin levels were within the normal range. Ultrasound of the neck, revealed a right lobe hypoechoic nodule 0.5 × 0.4 cm classified as TI-RADS 4. As well as a left lobe lesion measuring 2.3 × 1.7 cm, classified as TI-RADS 5 (Figs 1 and 2). Fine needle aspiration of the left lobe nodule confirmed the diagnosis of papillary thyroid carcinoma.
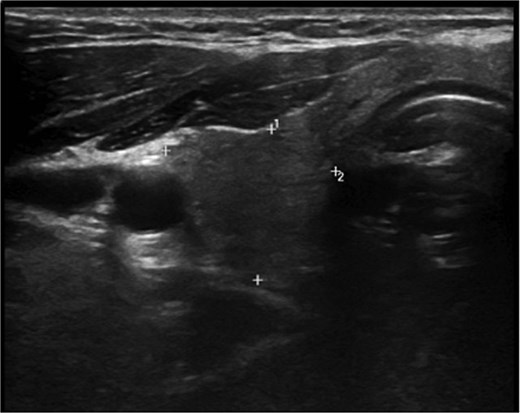
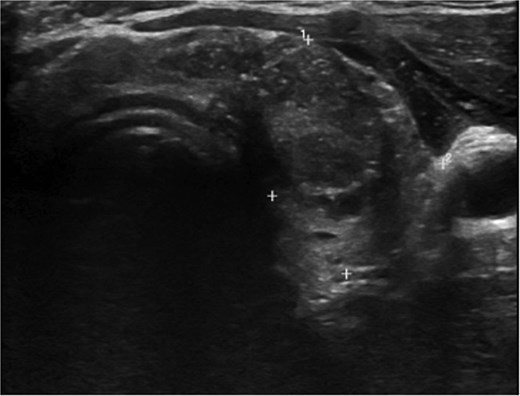
He was scheduled for a total thyroidectomy and central neck dissection the following week. Postoperatively, he was started on oral calcium carbonate, alpha- calcidiol, and thyroxine. The patient developed peripheral and perioral numbness on the third day; this was managed with IV calcium and magnesium, as well as increasing the dose of his oral calcium carbonate and alpha- calcidiol. The patient was discharged after 1 week.
The final pathology report revealed a 2.8 cm unifocal papillary thyroid carcinoma, Warthin-like variant, in the left lobe, with lymphatic invasion, one metastatic Level VI lymph node (1/5, 1 mm deposit), no extrathyroidal extension or extra nodal involvement, and uninvolved margins (closest <1 mm posteriorly), alongside concurrent Hashimoto’s thyroiditis and thyroid follicular nodular disease, classified as pT2 pN1a (AJCC 8th edition).
He was referred to thyroid oncology and continues to be in remission 6 months post op. His follow up labs are shown below in Tables 1 and 2.
| TSH reference (0.2700–4.2000) uIU/ml . | T4 reference (12.00–22.00) pmol/L . | Corrected calcium reference (2.10–2.55) mmol/L . | Albumin (39.70–49.40) g/l . | Magnesium reference (0.7–1.10) mmol/L . | Phosphorus reference (0.81–1.45) mmol/L . | |
|---|---|---|---|---|---|---|
| Pre-operative | 2.0500 | 26.7 | 2.24 | 46.2 | 0.77 | 1.07 |
| Upon discharge | NA | NA | 2.1 | 44.7 | 0.93 | 1.77 |
| 1 week | 37.90 | NA | 1.9 | 38.1 | 0.87 | 1.85 |
| 2 weeks | NA | 16.30 | 2.78 | 46.3 | 0.99 | 1.55 |
| 1 month | 38.70 | 22.30 | 2.15 | 47.4 | 0.85 | 0.87 |
| 3 months | 32.00 | 15.80 | 2.16 | 46.2 | NA | 0.98 |
| TSH reference (0.2700–4.2000) uIU/ml . | T4 reference (12.00–22.00) pmol/L . | Corrected calcium reference (2.10–2.55) mmol/L . | Albumin (39.70–49.40) g/l . | Magnesium reference (0.7–1.10) mmol/L . | Phosphorus reference (0.81–1.45) mmol/L . | |
|---|---|---|---|---|---|---|
| Pre-operative | 2.0500 | 26.7 | 2.24 | 46.2 | 0.77 | 1.07 |
| Upon discharge | NA | NA | 2.1 | 44.7 | 0.93 | 1.77 |
| 1 week | 37.90 | NA | 1.9 | 38.1 | 0.87 | 1.85 |
| 2 weeks | NA | 16.30 | 2.78 | 46.3 | 0.99 | 1.55 |
| 1 month | 38.70 | 22.30 | 2.15 | 47.4 | 0.85 | 0.87 |
| 3 months | 32.00 | 15.80 | 2.16 | 46.2 | NA | 0.98 |
| TSH reference (0.2700–4.2000) uIU/ml . | T4 reference (12.00–22.00) pmol/L . | Corrected calcium reference (2.10–2.55) mmol/L . | Albumin (39.70–49.40) g/l . | Magnesium reference (0.7–1.10) mmol/L . | Phosphorus reference (0.81–1.45) mmol/L . | |
|---|---|---|---|---|---|---|
| Pre-operative | 2.0500 | 26.7 | 2.24 | 46.2 | 0.77 | 1.07 |
| Upon discharge | NA | NA | 2.1 | 44.7 | 0.93 | 1.77 |
| 1 week | 37.90 | NA | 1.9 | 38.1 | 0.87 | 1.85 |
| 2 weeks | NA | 16.30 | 2.78 | 46.3 | 0.99 | 1.55 |
| 1 month | 38.70 | 22.30 | 2.15 | 47.4 | 0.85 | 0.87 |
| 3 months | 32.00 | 15.80 | 2.16 | 46.2 | NA | 0.98 |
| TSH reference (0.2700–4.2000) uIU/ml . | T4 reference (12.00–22.00) pmol/L . | Corrected calcium reference (2.10–2.55) mmol/L . | Albumin (39.70–49.40) g/l . | Magnesium reference (0.7–1.10) mmol/L . | Phosphorus reference (0.81–1.45) mmol/L . | |
|---|---|---|---|---|---|---|
| Pre-operative | 2.0500 | 26.7 | 2.24 | 46.2 | 0.77 | 1.07 |
| Upon discharge | NA | NA | 2.1 | 44.7 | 0.93 | 1.77 |
| 1 week | 37.90 | NA | 1.9 | 38.1 | 0.87 | 1.85 |
| 2 weeks | NA | 16.30 | 2.78 | 46.3 | 0.99 | 1.55 |
| 1 month | 38.70 | 22.30 | 2.15 | 47.4 | 0.85 | 0.87 |
| 3 months | 32.00 | 15.80 | 2.16 | 46.2 | NA | 0.98 |
| Thyroglobulin antibodies . | Thyroglobulin reference (3.50–77.00) ng/mL . | |
|---|---|---|
| 1 month | 19.19 | 0.12 |
| 3 months | NA | 0.08 |
| Thyroglobulin antibodies . | Thyroglobulin reference (3.50–77.00) ng/mL . | |
|---|---|---|
| 1 month | 19.19 | 0.12 |
| 3 months | NA | 0.08 |
| Thyroglobulin antibodies . | Thyroglobulin reference (3.50–77.00) ng/mL . | |
|---|---|---|
| 1 month | 19.19 | 0.12 |
| 3 months | NA | 0.08 |
| Thyroglobulin antibodies . | Thyroglobulin reference (3.50–77.00) ng/mL . | |
|---|---|---|
| 1 month | 19.19 | 0.12 |
| 3 months | NA | 0.08 |
Discussion
The WL-PTC is considered a rare type of PTC. It was discovered in 1995 by Apel et al. [8]. Clinically, patients often present with a painless mass on the neck, similar to other thyroid malignancies [9]. Many cases show an association between WL-PTC and chronic lymphocytic thyroiditis; however, the exact mechanism and association has not been fully understood. Further research and investigations have suggested possible links between these two conditions [10]. WL-PTC has distinct histopathological features, including oncocytic cells and a lymphocytic stroma [7]. Differentiating the WL-PTC from other thyroid conditions, such as Hashimoto’s thyroiditis, Hurthle cell tumors, and the tall cell variant of PTC, is essential for an accurate diagnosis. A key feature of WL-PTC is the presence of oncocytic cells that display classic PTC nuclear characteristics, accompanied by a dense lymphoplasmacytic infiltrate. Immunohistochemical staining plays a crucial role in confirming the diagnosis, with WL-PTC typically showing positive staining for markers like thyroglobulin and TTF-1 [11]. WL-PTC has good clinical outcomes, and as a rare type of cancer, it is generally considered to be similar to or less aggressive than classical PTC, with long-term survival rates reaching 90% of 20 years survival rates [5, 12]. Diagnosis of WL-PTC is achieved through fine needle aspiration cytology [7, 8]. Management includes total thyroidectomy, followed by regular follow up to monitor for potential recurrence [9].
Conclusion
WL-PTC is a rare type of PTC. Given its histological similarity to Hashimoto’s thyroiditis, accurate diagnosis through histopathological and immunohistochemical analysis is required for early detection and management to achieve optimal patient outcomes.
Acknowledgements
The authors acknowledge the institutional support provided by King Saud University Medical City (KSUMC), Riyadh, Saudi Arabia, which facilitated the clinical evaluation and diagnostic procedures necessary for this study. This research received no external funding, and the authors declare no conflicts of interest.
Conflict of interest statement
None declared.
Funding
None declared.
References
Limaiem F, Rehman A, Mazzoni T.
Hryshchyshyn A, Bahrii A, Botsun P, et al.



